Abstract
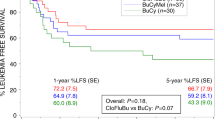
The optimal conditioning regimen for high-risk myelodysplastic syndrome (MDS) patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains elusive. This study aimed to explore the anti-leukemic efficacy and toxicity of Decitabine (Dec, 20 mg/m2/day, day −11 to −7) intensified BUCY2 vs. traditional regimen in high-risk MDS population. We retrospectively evaluated 93 consecutive high-risk MDS patients undergoing allo-HSCT in our institution, comparing discrepancies in clinical characteristics and outcomes between cases using Dec-intensified BUCY2 (n = 52) and traditional BUCY2 regimen (n = 41). Three-year cumulative incidence of relapse after Dec-intensified BUCY2 conditioning was remarkably lower than that of patients using BUCY2 regimen (20.2% vs. 39.0%, p = 0.034). Overall survival and disease-free survival at 3 years for Dec-intensified BUCY2 group were 70.2% and 64.9%, respectively, which were significantly improved when compared with BUCY2 group (51.1% and 43.9%, p = 0.031 and p = 0.027). Furthermore, overall survival and disease-free survival for MDS cases receiving cytoreduction therapy were dramatically better than patients in non-cytoreduction group (p = 0.041, p = 0.047). In summary, the Dec-intensified conditioning regimen could be effective and feasible, providing prominent recurrence control with moderate toxicity for high-risk MDS patients. These patients might also benefit from pre-transplant cytoreductive therapeutic schedules. Larger randomized controlled trials are still needed to further confirm these conclusions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Yoo JW, Im HJ, Kim H, Koh KN, Kang SH, Min SY, et al. Improved outcomes of allogeneic hematopoietic stem cell transplantation including haploidentical transplantation for childhood myelodysplastic syndrome. Bone Marrow Transpl. 2020;55:1595–603.
Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transpl. 2009;15:30–8.
Yahng SA, Kim M, Kim TM, Jeon YW, Yoon JH, Shin SH, et al. Better transplant outcome with pre-transplant marrow response after hypomethylating treatment in higher-risk MDS with excess blasts. Oncotarget. 2017;8:12342–54.
Della Porta MG, Jackson CH, Alessandrino EP, Rossi M, Bacigalupo A, van Lint MT, et al. Decision analysis of allogeneic hematopoietic stem cell transplantation for patients with myelodysplastic syndrome stratified according to the revised International Prognostic Scoring System. Leukemia. 2017;31:2449–57.
Schuler E, Boughoufala S, Rautenberg C, Nachtkamp K, Dienst A, Fenk R, et al. Relapse patterns and treatment strategies in patients receiving allogeneic hematopoietic stem cell transplantation for myeloid malignancies. Ann Hematol. 2019;98:1225–35.
Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions-a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transpl. 2015;21:653–60.
Zhang R, Lu X, Wang H, You Y, Zhong Z, Zang S, et al. Idarubicin-intensified hematopoietic cell transplantation improves relapse and survival of high-risk acute leukemia patients with minimal residual disease. Biol Blood Marrow Transpl. 2019;25:47–55.
Zhang R, Shi W, Wang HF, You Y, Zhong ZD, Li WM, et al. Idarubicin-intensified haploidentical HSCT with GvHD prophylaxis of ATG and basiliximab provides comparable results to sibling donors in high-risk acute leukemia. Bone Marrow Transpl. 2017;52:1253–60.
Fang J, Zhang R, Wang H, Hong M, Wu Q, Nie D, et al. Idarubicin-intensified BUCY2 conditioning regimen improved survival in high-risk acute myeloid, but not lymphocytic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective comparative study. Leuk Res. 2016;46:61–8.
Wu Q, Zhang R, Wang H, You Y, Zhong Z, Hong M, et al. Comparison of outcomes of idarubicin intensified TBI-CY and traditional TBI-CY conditioning regimen for high-risk acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: A single center experience. Leuk Res. 2015;39:1192–200.
Ogawa S. Genetics of MDS. Blood. 2019;133:1049–59.
Stosch JM, Heumüller A, Niemöller C, Bleul S, Rothenberg-Thurley M, Riba J, et al. Gene mutations and clonal architecture in myelodysplastic syndromes and changes upon progression to acute myeloid leukaemia and under treatment. Br J Haematol. 2018;182:830–42.
Zhou X, Mei C, Zhang J, Lu Y, Lan J, Lin S, et al. Epigenetic priming with decitabine followed by low dose idarubicin and cytarabine in acute myeloid leukemia evolving from myelodysplastic syndromes and higher-risk myelodysplastic syndromes: a prospective multicenter single-arm trial. Hematol Oncol. 2020;38:531–40.
Odenike O, Onida F, Padron E. Myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms: an update on risk stratification, molecular genetics, and therapeutic approaches including allogeneic hematopoietic stem cell transplantation. Am Soc Clin Oncol Educ Book. 2015;35:e398–412.
Wang QY, Li Y, Liang ZY, Yin Y, Liu W, Wang Q, et al. Decitabine-containing conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with intermediate- and high-risk myelodysplastic syndrome/acute myeloid leukemia: potential decrease in the incidence of acute graft versus host disease. Cancer Manag Res. 2019;11:10195–203.
Alessandrino EP, Della Porta MG, Pascutto C, Bacigalupo A, Rambaldi A. Should cytoreductive treatment be performed before transplantation in patients with high-risk myelodysplastic syndrome? J Clin Oncol. 2013;31:2761–2.
Damaj G, Mohty M, Robin M, Michallet M, Chevallier P, Beguin Y, et al. Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study by the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Biol Blood Marrow Transpl. 2014;20:1349–55.
Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J Clin Oncol. 2012;30:4533–40.
Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transpl. 2012;18:1211–8.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.
Guièze R, Damaj G, Pereira B, Robin M, Chevallier P, Michallet M, et al. Management of myelodysplastic syndrome relapsing after allogeneic hematopoietic stem cell transplantation: a study by the French Society of Bone Marrow Transplantation and Cell Therapies. Biol Blood Marrow Transpl. 2016;22:240–7.
Wu D, Du X, Jin J, Xiao Z, Shen Z, Shao Z, et al. Decitabine for treatment of myelodysplastic syndromes in Chinese patients: an open-label, phase-3b study. Adv Ther. 2015;32:1140–59.
Welch JS, Petti AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–36.
Sidaway P. Haematological cancer: TP53 mutations sensitize to decitabine. Nat Rev Clin Oncol. 2017;14:72.
Chang CK, Zhao YS, Xu F, Guo J, Zhang Z, He Q, et al. TP53 mutations predict decitabine-induced complete responses in patients with myelodysplastic syndromes. Br J Haematol. 2017;176:600–8.
Flotho C, Sommer S, Lübbert M. DNA-hypomethylating agents as epigenetic therapy before and after allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes and juvenile myelomonocytic leukemia. Semin Cancer Biol. 2018;51:68–79.
Cruijsen M, Hobo W, van der Velden WJFM, Bremmers MEJ, Woestenenk R, Bär B, et al. Addition of 10-day decitabine to fludarabine/total body irradiation conditioning is feasible and induces tumor-associated antigen-specific T cell responses. Biol Blood Marrow Transpl. 2016;22:1000–8.
Cao YG, He Y, Zhang SD, Liu ZX, Zhai WH, Ma QL, et al. Conditioning regimen of 5-day decitabine administration for allogeneic stem cell transplantation in patients with myelodysplastic syndrome and myeloproliferative neoplasms. Biol Blood Marrow Transpl. 2020;26:285–91.
Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–7.
Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from the Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14:145.
Sobecks RM, Rybicki L, Yurch M, Kalaycio M, Dean R, Andresen S, et al. Intravenous compared with oral busulfan as preparation for allogeneic hematopoietic progenitor cell transplantation for AML and MDS. Bone Marrow Transpl. 2012;47:633–8.
Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transpl. 2016;51:232–40.
Valdez BC, Li Y, Murray D, Corn P, Champlin RE, Andersson BS. 5-Aza-2’-deoxycytidine sensitizes busulfan-resistant myeloid leukemia cells by regulating expression of genes involved in cell cycle checkpoint and apoptosis. Leuk Res. 2010;34:364–72.
Valdez BC, Tang X, Li Y, Murray D, Liu Y, Popat U, et al. Epigenetic modification enhances the cytotoxicity of busulfan and 4-hydroperoxycyclophosphamide in AML cells. Exp Hematol. 2018;67:49–59.
Schroeder T, Wegener N, Lauseker M, Rautenberg C, Nachtkamp K, Schuler E, et al. Comparison between upfront transplantation and different pretransplant cytoreductive treatment approaches in patients with high-risk myelodysplastic syndrome and secondary acute myelogenous leukemia. Biol Blood Marrow Transpl. 2019;25:1550–9.
Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–64.
Acknowledgements
The present work was supported and funded by the National Natural Science Foundation of China (Nos. 81974003, 81900181, 81370668), Key Scientific Research Project of Henan University (No. 20A320021) and Collaborative Innovation Center of Hematology of China.
Author information
Authors and Affiliations
Contributions
L-HX and WS conceived the protocol; RZ analyzed, interpreted the data and wrote the manuscript; XL, LVT and HY carried out the statistical analysis; H-FW, YY and Z-DZ revised the manuscript, and all authors critically reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
We have reviewed the final version of the manuscript and approved it for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, R., Lu, X., Tang, L.V. et al. Comparative analysis of Decitabine intensified BUCY2 and BUCY2 conditioning regimen for high-risk MDS patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 57, 1063–1071 (2022). https://doi.org/10.1038/s41409-022-01645-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01645-2