Abstract
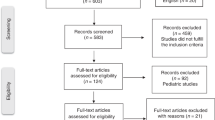
Hemophagocytic lymphohistiocytosis (HLH; hemophagocytic syndrome) is a rare syndrome of potentially fatal, uncontrolled hyperinflammation. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is indicated in primary, recurrent or progressive HLH, but information about its outcomes in the adult population is limited. We obtained data about 87 adult (≥18 years of age) patients retrospectively reported to the EBMT. The median survival time was 13.9 months. The three and five-year overall survival (OS) was 44% (95% CI 33–54%). Among 39 patients with a follow-up longer than 15 months, only three died. Relapse rate was 21% (95% CI 13–30%), while NRM reached 36% (95% CI 25–46%). Younger patients (<30 years of age) had better prognosis, with an OS of 59% (95% CI 45–73%) at three and five years vs 23% (95% CI 8–37%) for older ones. No difference in survival between reduced and myeloablative conditioning was found. To our knowledge, this is the largest report of adult HLH patients who underwent allo-HSCT. Patients who survive the first period after this procedure can expect a long disease-free survival. Both reduced intensity and myeloablative conditioning have therapeutic potential in adult HLH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Janka GE, Lehmberg K. Hemophagocytic syndromes-an update. Blood Rev. 2014;28:135–42.
Jordan MB, Allen CE, Greenberg J, Henry M, Hermiston ML, Kumar A, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:1–12.
Cetica V, Sieni E, Pende D, Danesino C, De Fusco C, Locatelli F, et al. Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. J Allergy Clin Immunol. 2016;137:188–.e4.
Nagafuji K, Nonami A, Kumano T, Kikushige Y, Yoshimoto G, Takenaka K, et al. Perforin gene mutations in adult-onset hemophagocytic lymphohistiocytosis. Haematologica. 2007;92:978–81.
Sieni E, Cetica V, Piccin A, Gherlinzoni F, Sasso FC, Rabusin M et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0044649.
Fischer A, Cerf-Bensussan N, Blanche S, Le Deist F, Bremard-Oury C, Leverger G, et al. Allogeneic bone marrow transplantation for erythrophagocytic lymphohistiocytosis. J Pediatr. 1986;108:267–70.
Henter JI, Aricò M, Egeler RM, Elinder G, Favara BE, Filipovich AH, et al. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. HLH study Group of the Histiocyte Society. Med Pediatr Oncol. 1997;28:342–7.
Henter J-I, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31.
Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577–84.
Bergsten E, Horne AC, Aricó M, Astigarraga I, Egeler RM, Filipovich AH, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: Long-Term results of the cooperative HLH-2004 study. Blood. 2017;130:2728–38.
Bergsten E, Horne A, Hed Myrberg I, Aricó M, Astigarraga I, Ishii E, et al. Stem cell transplantation for children with hemophagocytic lymphohistiocytosis: results from the HLH-2004 study. Blood Adv. 2020;4:3754–66.
Marsh RA, Vaughn G, Kim M-O, Li D, Jodele S, Joshi S, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824–31.
Allen CE, Marsh R, Dawson P, Bollard CM, Shenoy S, Roehrs P, et al. Reduced-intensity conditioning for hematopoietic cell transplant for HLH and primary immune deficiencies. Blood. 2018;132:1438–51.
Nikiforow S. Finding ‘intermediate’ ground in transplant and HLH. Blood. 2018;132:1361–3.
Marsh RA, Haddad E. How i treat primary haemophagocytic lymphohistiocytosis. Br J Haematol. 2018;182:185–99.
Scott Baker K, Jordan MB. Hematopoietic cell transplantation and novel therapies in hemophagocytic lymphohistiocytosis. In: Abla O, Janka G (eds). Histiocytic Disorders. Springer International Publishing: Cham, 2018, 265–74.
Lehmberg K, Moshous D, Booth C. Haematopoietic stem cell transplantation for primary haemophagocytic lymphohistiocytosis. Front Pediatr. 2019;7. https://doi.org/10.3389/fped.2019.00435.
Felber M, Steward CG, Kentouche K, Fasth A, Wynn RF, Zeilhofer U, et al. Targeted busulfan-based reduced-intensity conditioning and HLA-matched HSCT cure hemophagocytic lymphohistiocytosis. Blood Adv. 2020;4:1998–2010.
Greental Ness Y, Kuperman AA, Stein J, Yacobovich J, Even-Or E, Zaidman I et al. Improved transplant outcomes with myeloablative conditioning for hemophagocytic lymphohistiocytosis in HLA-matched and mismatched donors: a national multicenter retrospective study. Bone Marrow Transplant. 2021. https://doi.org/10.1038/s41409-021-01290-1.
La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;: blood.2018894618.
Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908–14.
La Rosée P. Treatment of hemophagocytic lymphohistiocytosis in adults. Hematol Am Soc Hematol Educ Program. 2015;2015:190–6.
La Rosée P, Machowicz R HLH in Adults. In: Histiocytic Disorders. Springer International Publishing: Cham, 2018, 275–90.
Fu L, Wang J, Wei N, Wu L, Wang Y, Huang W, et al. Allogeneic hematopoietic stem-cell transplantation for adult and adolescent hemophagocytic lymphohistiocytosis: a single center analysis. Int J Hematol. 2016;104:628–35.
Park H-S, Lee J-H, Lee J-H, Choi E-J, Ko S-H, Seol M et al. Fludarabine/melphalan 100 mg/m2 conditioning therapy followed by allogeneic hematopoietic cell transplantation for adult patients with secondary hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2018. https://doi.org/10.1016/j.bbmt.2018.11.032.
Li Z, Wang Y, Wang J, Zhang J, Wang Z. Haploidentical hematopoietic stem cell transplantation for adult patients with Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2018;59:77–84.
Nikiforow S, Korman S, Eapen M, Antin JH. Outcomes after allogeneic stem cell transplantation in adults for histiocytic disorders including hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transpl. 2014;20:S243.
Schram AM, Comstock P, Campo M, Gorovets D, Mullally A, Bodio K, et al. Haemophagocytic lymphohistiocytosis in adults: a multicentre case series over 7 years. Br J Haematol. 2016;172:412–9.
Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220–4.
Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58–65.
Jin Z, Wang Y, Wang J, Zhang J, Wu L, Gao Z, et al. Primary hemophagocytic lymphohistiocytosis in adults: the utility of family surveys in a single-center study from China. Orphanet J Rare Dis. 2018;13:17.
Sato E, Ohga S, Kuroda H, Yoshiba F, Nishimura M, Nagasawa M, et al. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83:721–7.
Lehmberg K, Albert MH, Beier R, Beutel K, Gruhn B, Kröger N, et al. Treosulfan-based conditioning regimen for children and adolescents with hemophagocytic lymphohistiocytosis. Haematologica. 2014;99:180–4.
Naik S, Eckstein O, Sasa G, Heslop HE, Krance RA, Allen C et al. Incorporation of thiotepa in a reduced intensity conditioning regimen may improve engraftment after transplant for HLH. Br J Haematol. 2020;188. https://doi.org/10.1111/bjh.16370.
Marsh R, Grimley M, Bleesing J, Jordan M, Filipovich AH. Adolescents and young adults with hemophagocytic lymphohistiocytosis who undergo allogeneic hematopoietic cell transplantation are at increased risk of mortality compared to younger patients. Blood. 2013;122:2087 LP–2087.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Tanaka Y, Kurosawa S, Tajima K, Tanaka T, Ito R, Inoue Y, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2016;51:553–9.
Messina C, Zecca M, Fagioli F, Rovelli A, Giardino S, Merli P et al. Outcomes of children with hemophagocytic lymphohistiocytosis given allogeneic hematopoietic stem cell transplantation in Italy. Biol Blood Marrow Transplant. 2018. https://doi.org/10.1016/j.bbmt.2018.01.022.
Baker KS, Filipovich AH, Gross TG, Grossman WJ, Hale GA, Hayashi RJ, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transpl. 2008;42:175–80.
Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122:3359–64.
Liu J, Kong J, Chang YJ, Chen H, Chen YH, Han W, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect. 2015;21:1121.e9–15.
Hartz B, Marsh R, Rao K, Henter J-I, Jordan M, Filipovich L, et al. The minimum required level of donor chimerism in hereditary hemophagocytic lymphohistiocytosis. Blood. 2016;127:3281–90.
Pagel J, Beutel K, Lehmberg K, Koch F, Maul-Pavicic A, Rohlfs A-K, et al. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5). Blood. 2012;119:6016–24.
Author information
Authors and Affiliations
Contributions
RM conceived and designed the study, analyzed and interpreted data, designed the questionnaire and drafted the manuscript, NK designed the study, analyzed and interpreted data and edited the manuscript, FS designed the questionnaire, provided clinical data and edited the manuscript, WWJ assisted with the study design and data interpretation and edited the manuscript, DJE and LdW performed statistical analyses and edited the manuscript, HJB performed data collection and edited the manuscript, CI, HE, XP, SvD, EN, JEJ, GK, MZ, RA, AG, JF, JLDM, FB, GMQ, SL, PSR, MT, PL, MC, MA, GE, KC, KH, KL, SS provided clinical data and edited the manuscript, AL, AG and IYA assisted with the study design and data interpretation and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Machowicz, R., Suarez, F., Wiktor-Jedrzejczak, W. et al. Allogeneic hematopoietic stem cell transplantation for adult HLH: a retrospective study by the chronic malignancies and inborn errors working parties of EBMT. Bone Marrow Transplant 57, 817–823 (2022). https://doi.org/10.1038/s41409-022-01634-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01634-5