Abstract
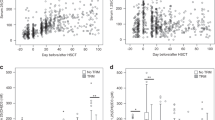
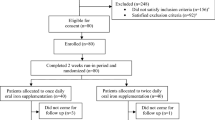
Vitamin D deficiency remains common among pediatric patients undergoing hematopoietic stem cell transplant (HSCT) despite both aggressive and standard of care strategies. This study examined the safety and efficacy of single high-dose oral vitamin D therapy (Stoss therapy) for treatment of vitamin D deficiency in HSCT recipients. Patients ages 1–21 years presenting for HSCT were randomized to receive either Stoss regimen plus weekly/daily supplementation or standard of care, per US Endocrine Society guidelines. Among the total 48 subjects, 22 (46%) were randomized to Stoss and 26 (54%) to control arms. Baseline 25-hydroxyvitamin D (25-OHD) levels were insufficient/deficient in total of 34 (71%) patients, without difference between treatment groups. The Stoss regimen was well tolerated and no toxicity was observed. At Day +30, mean 25-OHD levels were significantly higher (P = 0.04) with Stoss (42.3 ± 12 μg/l) compared to controls (35.6 ± 14.3 μg/l), and a higher proportion of Stoss patients had adequate vitamin D levels than controls (85% vs 65%). Stoss therapy is a safe and efficacious treatment option for vitamin D deficiency in children undergoing HSCT and may achieve sufficient levels more rapidly than standard of care. This trial was registered at www.clinicaltrials.gov as NCT03176849.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Campos DJ, Boguszewski CL, Funke VA, Bonfim CM, Kulak CA, Pasquini R, et al. Bone mineral density, vitamin D, and nutritional status of children submitted to hematopoietic stem cell transplantation. Nutrition. 2014;30:654–9.
Duncan CN, Vrooman L, Apfelbaum EM, Whitley K, Bechard L, Lehmann LE. 25-hydroxy vitamin D deficiency following pediatric hematopoietic stem cell transplant. Biol Blood Marrow Transpl. 2011;17:749–53.
Wallace G, Jodele S, Howell J, Myers KC, Teusink A, Zhao X, et al. Vitamin D deficiency and survival in children after hematopoietic stem cell transplant. Biol Blood Marrow Transpl. 2015;21:1627–31.
Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Vitamin D deficiency in pediatric hematopoietic stem cell transplantation patients despite both standard and aggressive supplementation. Biol Blood Marrow Transpl. 2016;22:1271–4.
Bechard LJ, Gordon C, Feldman HA, Venick R, Gura K, Guinan EC, et al. Bone loss and vitamin D deficiency in children undergoing hematopoietic cell transplantation. Pediatr Blood Cancer. 2015;62:687–92.
Beebe K, Magee K, McNulty A, Stahlecker J, Salzberg D, Miller H, et al. Vitamin D deficiency and outcomes in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2017;65:e26817.
Stein EM, Shane E. Vitamin D in organ transplantation. Osteoporos Int. 2011;22:2107–18.
Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–44.
von Bahr L, Blennow O, Alm J, Bjorklund A, Malmberg KJ, Mougiakakos D, et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transpl. 2015;50:1217–23.
Sproat L, Bolwell B, Rybicki L, Dean R, Sobecks R, Pohlman B, et al. Vitamin D level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transpl. 2011;17:1079–83.
Glotzbecker B, Ho VT, Aldridge J, Kim HT, Horowitz G, Ritz J, et al. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transpl. 2013;48:593–7.
Rosenblatt J, Bissonnette A, Ahmad R, Wu Z, Vasir B, Stevenson K, et al. Immunomodulatory effects of vitamin D: Implications for GVHD. Bone Marrow Transplant. 2010;45:1463–8.
Caballero-Velazquez T, Montero I, Sanchez-Guijo F, Parody R, Saldana R, Valcarcel D, et al. Immunomodulatory effect of vitamin D after allogeneic stem cell transplantation: results of a prospective multicenter clinical trial. Clin Cancer Res. 2016;22:5673–81.
Henden AS, Hill GR. Cytokines in graft-versus-host disease. J Immunol. 2015;194:4604–12.
Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1, 25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984;3:1451–5.
GO H. [Die Stossprophylaxe der rachitis]. Dtsch Med Wochenschr. 1938;64:1835–7. [Article in German].
Shah BRFL. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr Endocrinol Metab. 1994;125:487–90.
Cesur Y, Caksen H, Gündem A, Kirmi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16:1105–9.
Shepherd D, Belessis Y, Katz T, Morton J, Field P, Jaffe A. Single high-dose oral vitamin D3 (stoss) therapy-a solution to vitamin D deficiency in children with cystic fibrosis?. J Cyst Fibros. 2013;12:177–82.
Shepherd DDA, Leach ST, Lopez R, Messenger R, Woodhead HJ, Ledder O, et al. Single high-dose oral vitamin D3 therapy (Stoss): a solution to vitamin D deficiency in children with inflammatory bowel disease? J Pediatr Gastroenterol Nutr. 2015;61:411–4.
Koçyiğit CÇG, İnce G, Özkan EB, Dündar BN. Can Stoss therapy be used in children with vitamin D deficiency or insufficiency without rickets? J Clin Res Pediatr Endocrinol. 2017;9:150–5.
Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Single ultra-high-dose cholecalciferol to prevent vitamin D deficiency in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2018;24:1856–60.
Michael F, Holick NCB, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Sylvia Christakos PD, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347:25–9.
Fukumoto S. Phosphate metabolism and vitamin D. Bonekey Rep. 2014;3:497. https://doi.org/10.1038/bonekey.2013.231.
Ilahi M, Armas LAG, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–91.
Davies JH, Evans BAJ, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90:373–8.
Acknowledgements
The authors thank research coordinators Kayla Burgett, Kara Kronemeyer, and Jessica Cline, who collected the data for the trial.
Funding
Grants from the Learners Research Fund, Phoenix Children’s Hospital and Valley Research Partnership Grant (P1201707), University of Arizona, provided support for the trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bodea, J., Beebe, K., Campbell, C. et al. Stoss therapy is safe for treatment of vitamin D deficiency in pediatric patients undergoing HSCT. Bone Marrow Transplant 56, 2137–2143 (2021). https://doi.org/10.1038/s41409-021-01294-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01294-x