Abstract
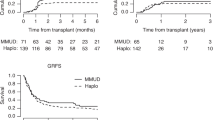
HLA haploidentical hematopoietic stem cell transplantation (HSCT), i.e., HSCT from a 1-HLA-haplotype-mismatched family donor, has been successfully performed even as a second transplantation for posttransplant relapse. Is the haploidentical the limit of HLA mismatches in HSCT? In order to explore the possibility of HLA-mismatched HSCT from family donors beyond haploidentical relatives, we conducted a prospective phase I/II study of 2-HLA-haplotype-mismatched HSCT (2-haplo-mismatch HSCT). We enrolled 30 patients with posttransplant relapse (acute myeloid leukemia: 18, acute lymphoblastic leukemia: 11, non-Hodgkin lymphoma: 1). 2-haplo-mismatch HSCT was performed as the second to sixth transplantations. The donors were siblings (n = 12), cousins (n = 16), and second cousins (n = 2). The conditioning regimen consisted of fludarabine, cytarabine, melphalan, low-dose anti-thymocyte globulin, and 3 Gy of total body irradiation. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus, methylprednisolone, and mycophenolate mofetil. All patients achieved neutrophil engraftment, except for a case of early death. The cumulative incidences of grades II–IV and III–IV acute GVHD were 36.7% and 16.7%, respectively. The overall survival at 1 year, relapse, and non-relapse mortality rates was 30.1%, 38.9%, and 44.3%, respectively. Considering the poor prognosis of posttransplant relapse, 2-haplo-mismatch HSCT can be an alternative option in a second or third transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34:721–7.
Lim ABM, Curley C, Fong CY, Bilmon I, Beligaswatte A, Purtill D, et al. Acute myeloid leukaemia relapsing after allogeneic haemopoietic stem cell transplantation: prognostic factors and impact of initial therapy of relapse. Intern Med J. 2018;48:276–85.
Choi EJ, Lee JH, Lee JH, Park HS, Ko SH, Seol M, et al. Treatment and clinical outcomes of patients relapsing after allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. Blood Res. 2018;53:288–93.
Ruutu T, de Wreede LC, van Biezen A, Brand R, Mohty M, Dreger P, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 2015;50:1542–50.
Spitzer B, Perales MA, Kernan NA, Prockop SE, Zabor EC, Webb N, et al. Second allogeneic stem cell transplantation for acute leukemia using a chemotherapy-only cytoreduction with clofarabine, melphalan, and thiotepa. Biol Blood Marrow Transplant. 2016;22:1449–54.
Vrhovac R, Labopin M, Ciceri F, Finke J, Holler E, Tischer J, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. Bone Marrow Transplant. 2016;51:186–93.
Weisdorf D. The role of second transplants for leukemia. Best Pract Res Clin Haematol. 2016;29:359–64.
Orti G, Sanz J, Bermudez A, Caballero D, Martinez C, Sierra J, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: a retrospective cohort on behalf of the Grupo Espanol de Trasplante Hematopoyetico. Biol Blood Marrow Transplant. 2016;22:584–8.
Taga T, Murakami Y, Tabuchi K, Adachi S, Tomizawa D, Kojima Y, et al. Role of second transplantation for children with acute myeloid leukemia following posttransplantation relapse. Pediatr Blood Cancer. 2016;63:701–5.
Aljasem HA, Messner HA, Lipton JH, Kim DDH, Viswabandya A, Thyagu S, et al. Outcome following second allogeneic hematopoietic cell transplantation: a single-center experience. Eur J Haematol. 2018;100:308–14.
Schneidawind C, Hagmaier V, Faul C, Kanz L, Bethge W, Schneidawind D. Second allogeneic hematopoietic cell transplantation enables long-term disease-free survival in relapsed acute leukemia. Ann Hematol. 2018;97:2491–500.
Yaniv I, Krauss AC, Beohou E, Dalissier A, Corbacioglu S, Zecca M, et al. Second hematopoietic stem cell transplantation for post-transplantation relapsed acute leukemia in children: a retrospective EBMT-PDWP study. Biol Blood Marrow Transplant. 2018;24:1629–42.
Lund TC, Ahn KW, Tecca HR, Hilgers MV, Abdel-Azim H, Abraham A, et al. Outcomes after second hematopoietic cell transplantation in children and young adults with relapsed acute leukemia. Biol Blood Marrow Transplant. 2019;25:301–6.
Gyurkocza B, Storb R, Chauncey TR, Maloney DG, Storer BE, Sandmaier BM. Second allogeneic hematopoietic cell transplantation for relapse after first allografts. Leuk lymphoma. 2019;60:1758–66.
Gorgeis J, Zhang X, Connor K, Brown S, Solomon SR, Morris LE, et al. T cell-replete HLA haploidentical donor transplantation with post-transplant cyclophosphamide is an effective salvage for patients relapsing after an HLA-matched related or matched unrelated donor transplantation. Biol Blood Marrow Transplant. 2016;22:1861–6.
Haen SP, Groh C, Schumm M, Backert L, Loffler MW, Federmann B, et al. Haploidentical hematopoietic cell transplantation using in vitro T cell depleted grafts as salvage therapy in patients with disease relapse after prior allogeneic transplantation. Ann Hematol. 2017;96:817–27.
Imus PH, Blackford AL, Bettinotti M, Iglehart B, Dietrich A, Tucker N, et al. Major histocompatibility mismatch and donor choice for second allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2017;23:1887–94.
Guo M, Hu KX, Yu CL, Sun QY, Qiao JH, Wang DH, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117:936–41.
Ringden O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30:447–55.
Ikegame K, Tanji Y, Kitai N, Tamaki H, Kawakami M, Fujioka T, et al. Successful treatment of refractory T-cell acute lymphoblastic leukemia by unmanipulated stem cell transplantation from an HLA 3-loci mismatched (haploidentical) sibling. Bone Marrow Transplant. 2003;31:507–10.
Ikegame K, Mukouchi C, Kunitomi A, Konaka Y, Kawakami M, Nishida S, et al. Successful treatment of bcr/abl-positive acute mixed lineage leukemia by unmanipulated bone marrow transplantation from an HLA-haploidentical (3-antigen-mismatched) cousin. Bone Marrow Transplant. 2003;31:1165–8.
Ogawa H, Ikegame K, Kawakami M, Tsuboi A, Kim EH, Hosen N, et al. Powerful graft-versus-leukemia effects exerted by HLA-haploidentical grafts engrafted with a reduced-intensity regimen for relapse following myeloablative HLA-matched transplantation. Transplantation. 2004;78:488–9.
Ogawa H, Ikegame K, Yoshihara S, Kawakami M, Fujioka T, Masuda T, et al. Unmanipulated HLA 2-3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1073–84.
Ogawa H, Ikegame K, Kaida K, Yoshihara S, Fujioka T, Taniguchi Y, et al. Unmanipulated HLA 2-3 antigen-mismatched (haploidentical) bone marrow transplantation using only pharmacological GVHD prophylaxis. Exp Hematol. 2008;36:1–8.
Ikegame K, Yoshida T, Yoshihara S, Daimon T, Shimizu H, Maeda Y, et al. Unmanipulated haploidentical reduced-intensity stem cell transplantation using fludarabine, busulfan, low-dose antithymocyte globulin, and steroids for patients in non-complete remission or at high risk of relapse: a prospective multicenter phase I/II study in Japan. Biol Blood Marrow Transplant. 2015;21:1495–505.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50 Suppl 2:S37–9.
Zhang YY, Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, et al. FLT3 internal tandem duplication does not impact prognosis after haploidentical allogeneic hematopoietic stem cell transplantation in AML patients. Bone Marrow Transplant. 2019;54:1462–70.
Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D, et al. Nonmyeloablative haploidentical bone marrow transplantation with post-transplantation cyclophosphamide for pediatric and young adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2017;23:325–32.
Rimando J, Slade M, DiPersio JF, Westervelt P, Gao F, Liu C, et al. HLA epitope mismatch in haploidentical transplantation is associated with decreased relapse and delayed engraftment. Blood Adv. 2018;2:3590–601.
Ikegame K, Kaida K, Yoshihara S, Yoshihara K, Ishii S, Inoue T, et al. Spousal hematopoietic stem cell transplantation. Int J Hematol. 2017;105:646–57.
Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–81.
Keil F, Prinz E, Moser K, Mannhalter C, Kalhs P, Worel N, et al. Rapid establishment of long-term culture-initiating cells of donor origin after nonmyeloablative allogeneic hematopoietic stem-cell transplantation, and significant prognostic impact of donor T-cell chimerism on stable engraftment and progression-free survival. Transplantation. 2003;76:230–6.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1.
Eizuru Y, Minematsu T, Minamishima Y, Ebihara K, Takahashi K, Tamura K, et al. Rapid diagnosis of cytomegalovirus infections by direct immunoperoxidase staining with human monoclonal antibody against an immediate-early antigen. Microbiol Immunol. 1991;35:1015–22.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Tamaki H, Ikegame K, Kawakami M, Fujioka T, Tsuboi A, Oji Y, et al. Successful engraftment of HLA-haploidentical related transplants using nonmyeloablative conditioning with fludarabine, busulfan and anti-T-lymphocyte globulin. Leukemia. 2003;17:2052–4.
Elmariah H, Kasamon YL, Zahurak M, Macfarlane KW, Tucker N, Rosner GL, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors. Biol Blood Marrow Transplant. 2018;24:1099–102.
Inamoto Y, Ito M, Suzuki R, Nishida T, Iida H, Kohno A, et al. Clinicopathological manifestations and treatment of intestinal transplant-associated microangiopathy. Bone Marrow Transplant. 2009;44:43–9.
Sorensen BS, Szer J, Shaw B, Korhonen M, Mengling T, Fechter M, et al. Inadvertent completely HLA-mismatched allogeneic unrelated bone marrow transplant: lessons learned. Bone Marrow Transplant. 2016;51:1016–8.
Sorensen CD, Moller BK, Olesen G, Hokland P, Hokland M. Complete donor chimerism following 0/10 HLA-mismatched unrelated donor allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018;53:1578–82.
Maeda Y, Ugai T, Kondo E, Ikegame K, Murata M, Uchida N, et al. HLA discrepancy between graft and host rather than that graft and first donor impact the second transplant outcome. Haematologica. 2019;104:1055–61.
Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204.
Ikegame K, Kaida K, Yoshihara S, Fujiwara M, Taniguchi K, Kato R, et al. Feasibility of unmanipulated haploidentical stem cell transplantation using standard GVHD prophylaxis for HLA-homozygous patients. Int J Hematol. 2012;96:101–8.
Kanda J, Ikegame K, Fuji S, Kurokawa M, Kanamori H, Fukuda T, et al. Haploidentical and matched sibling donor hematopoietic cell transplantation for patients with HLA-homozygous haplotypes. Biol Blood Marrow Transplant. 2016;22:2031–7.
Petrus MJ, Williams JF, Eckhaus MA, Gress RE, Fowler DH. An immunoablative regimen of fludarabine and cyclophosphamide prevents fully MHC-mismatched murine marrow graft rejection independent of GVHD. Biol Blood Marrow Transplant. 2000;6:182–9.
Dankers MK, Witvliet MD, Roelen DL, de Lange P, Korfage N, Persijn GG, et al. The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched human leukocyte antigens. Transplantation. 2004;77:1236–9.
Heemskerk MB, Roelen DL, Dankers MK, van Rood JJ, Claas FH, Doxiadis II, et al. Allogeneic MHC class I molecules with numerous sequence differences do not elicit a CTL response. Hum Immunol. 2005;66:969–76.
Heemskerk MB, Cornelissen JJ, Roelen DL, van Rood JJ, Claas FH, Doxiadis II, et al. Highly diverged MHC class I mismatches are acceptable for haematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:193–200.
Huo MR, Pei XY, Li D, Chang YJ, Xu LP, Zhang XH, et al. Impact of HLA allele mismatch at HLA-A, -B, -C, -DRB1, and -DQB1 on outcomes in haploidentical stem cell transplantation. Bone Marrow Transplant. 2018;53:600–8.
Mariotti J, Devillier R, Bramanti S, Sarina B, Furst S, Granata A, et al. T cell-replete haploidentical transplantation with post-transplantation cyclophosphamide for hodgkin lymphoma relapsed after autologous transplantation: reduced incidence of relapse and of chronic graft-versus-host disease compared with HLA-identical related donors. Biol Blood Marrow Transplant. 2018;24:627–32.
Sano H, Mochizuki K, Akaihata M, Kobayashi S, Ohto H, Kikuta A. T-cell-rich HLA-haploidentical hematopoietic stem cell transplantation for relapsed/refractory pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia without posttransplant tyrosine kinase inhibitor therapy. Pediatr Blood Cancer. 2017;64. https://doi.org/10.1002/pbc.26242.
Canaani J, Labopin M, Huang XJ, Arcese W, Ciceri F, Blaise D, et al. T-cell replete haploidentical stem cell transplantation attenuates the prognostic impact of FLT3-ITD in acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Am J Hematol. 2018;93:736–44.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–88.
Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115:3158–61.
Sano H, Mochizuki K, Kobayashi S, Ono S, Ikeda K, Ohto H, et al. Two occurrences of leukemia relapse due to mismatched hla loss after haploidentical stem cell transplantation from different family donors with kir ligand mismatch. J Pediatr Hematol/Oncol. 2020;42:e104–e106.
Onoe K, Gotohda T, Nishihori H, Aranami T, Iwabuchi C, Iclozan C, et al. Positive and negative selection of T cell repertoires during differentiation in allogeneic bone marrow chimeras. Transpl Immunol. 2003;12:79–88.
Acknowledgements
The authors would like to thank Ms. Ryoko Ishimoto for help with data management and Ms. Kimiko Yamamoto for excellent laboratory support. We also thank all physicians, nurses, pharmacists, physical therapists, and support personnel for their care of the patients in this study.
Funding
This work was supported in part by Health and Labour Sciences Research Grants for Clinical Cancer Research from the Ministry of Health, Labour and Welfare, Japan, Grants-in aid for Scientific Research (18K08376), and a Grant-in-Aid for Researchers, Hyogo College of Medicine.
Author information
Authors and Affiliations
Contributions
KI designed the study, analyzed data, wrote, and edited the paper. KK, KF, YO, KY, SY, SI, SF, TY, AM, SM, MT, and TI treated patients. MO, HT, HO, and YF supported clinical management.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ikegame, K., Kaida, K., Fukunaga, K. et al. Allogeneic hematopoietic stem cell transplantation from a 2-HLA-haplotype-mismatched family donor for posttransplant relapse: a prospective phase I/II study. Bone Marrow Transplant 56, 70–83 (2021). https://doi.org/10.1038/s41409-020-0980-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0980-8