Abstract
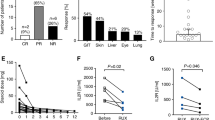
We compared three fludarabine-based regimens for systemic sclerosis patients with a high-risk cardiac phenotype that according to EBMT criteria would be a contraindication for a high-dose cyclophosphamide (200 mg/kg) transplant regimen. All three regimens included fludarabine, ATG, and cyclophosphamide (60 mg/kg), while two regimens also included rituximab with or without IVIG. Treatment related mortality (TRM) was 2.4%. The mean number of days of neutropenia (ANC < 500) was 5.2, the mean number of platelet and red blood cell transfusions was 0.3 and 1.85, respectively. Skin score, forced vital capacity (FVC), and total lung capacity (TLC) improved with all three regimens. For patients whose regimen did not include rituximab versus those that included rituximab, 1-year overall relapse rate was higher 36% (5/14) versus 3.6% (1 of 28) (p = 0.01), secondary autoimmune diseases were higher 21% (3/14) versus 0% (0/28) (p = 0.03), and upper respiratory tract infections were higher 28% (4/14) versus 3.6% (1/28) (p = 0.04). In this safety study, a fludarabine-based regimen was relatively safe with a TRM of 2.4% and a neutropenic interval of only 5.2 days in systemic sclerosis patients with a high-risk cardiac phenotype. The addition of rituximab decreased 1-year relapse rate, risk of late secondary autoimmune diseases, and upper-respiratory tract infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Burt RK, Oliveira MC, Shah SJ, Moraes DA, Simoes B, Gheorghiade M, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet. 2013;381:1116–24.
Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378:498–506.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014;311:2490–8.
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. 2018;378:35–47.
Burt RK, Shah SJ, Gheorghiade M, Ruderman E, Schroeder J. Hematopoietic stem cell transplantation for systemic sclerosis: if you are confused, remember: “it is a matter of the heart”. J Rheumatol. 2012;39:206–9.
Burt RK, Shah SJ, Schroeder J, Oliveira MC, Moraes DA, Simoes B, et al. Autologous HSCT for systemic sclerosis—authors’ Reply. Lancet. 2013;381(Jun):2080–1.
Burt RK, Oliveira MC, Shah SJ. Cardiac assessment before stem cell transplantation for systemic sclerosis. JAMA 2014;312:1803.
Farge D, Burt RK, Oliveira MC, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transpl. 2017;52:1495–503. https://doi.org/10.1038/bmt.2017.56. Epub 2017 May 22.
Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis. 2017;76:1897–905.
El-Gendy H, Shohdy KS, Maghraby GG, Abadeer K, Mahmoud M. Gastric antral vascular ectasia in systemic sclerosis: Where do we stand? Int J Rheum Dis. 2017;20:2133–9.
Ross L, Prior D, Proudman S, Vacca A, Baron M, Nikpour M. Defining primary systemic sclerosis heart involvement: a scoping literature review. Semin arthritis rheumatism. 2019;48:874–87.
Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. a cause of cardiac dysfunction. Circulation 1976;53:483–90.
Scully PR, Bastarrika G, Moon JC, Treibel TA. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. 2018;20:15.
Burt RK, Farge D. Systemic sclerosis: autologous HSCT is efficacious, but can we make it safer. Nat Rev Rheumatol. 2018;14:189–91.
Hegde UP, Wilson WH, White T, Cheson BD. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002;100:2260–2.
Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ, et al. Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann Rheum Dis. 2019;78:979–87.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical trial registry: ClinicalTrials.gov Identifier: NCT03593902
Rights and permissions
About this article
Cite this article
Burt, R.K., Han, X., Quigley, K. et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transplant 56, 50–59 (2021). https://doi.org/10.1038/s41409-020-0978-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0978-2
This article is cited by
-
Patients with systemic sclerosis and low CD4 numbers after autologous stem cell transplantation have a favorable outcome
Arthritis Research & Therapy (2024)
-
State-of-the-art evidence in the treatment of systemic sclerosis
Nature Reviews Rheumatology (2023)
-
Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2022)
-
Successful lymphoablative autologous haematopoietic stem cell transplantation in three cases of severe autoimmune disease despite reduced dose cyclophosphamide conditioning—do we need 200 mg/kg cyclophosphamide?
Bone Marrow Transplantation (2022)
-
Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022
Bone Marrow Transplantation (2022)