Abstract
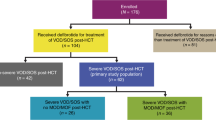
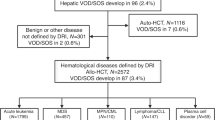
Hepatic veno-occlusive disease (VOD) is a potentially fatal complication following hematopoietic stem cell transplantation (HSCT). We evaluated in prospective analysis the usefulness of the pediatric EBMT criteria for VOD diagnosis and their presumable impact on cost effectiveness and patients’ outcome. Study included all 282 HSCT procedures performed in Department of Pediatric Hematology/Oncology and BMT in Wrocław between January 2016 and March 2019. Data were compared with previous VOD research conducted in our center before year 2016. Twenty-five (8.9%) patients (median age 3.5 years) were diagnosed with VOD. Duration of defibrotide (DF) administration varied from 4 to 34 days (median: 16.5), with 96% response rate. Overall survival was 88%. If applying Baltimore and modified Seattle criteria, VOD incidence was 2.13% and 5.7%, respectively. Median diagnosis delay based on modified Seattle criteria was 3 days. Before 2016, VOD incidence was 4.9%, with 74% DF response rate (p = 0.033) and 56.2% OS (p = 0.008). After implementing new criteria length of hospitalization for VOD patients decreased by median of 12 days (p = 0.009). Earlier VOD diagnosis, facilitated by EBMT criteria, resulting in implementing immediate treatment significantly improved patients’ outcome. Furthermore, it allows shortening of DF administration and minimizes length of hospital stay.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4:332–46.
Cesaro S, Spiller M, Sartori MT, Perusso M, Saggiorato G, Bisogno G. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer. 2011;57:258–61.
Cefalo MG, Maurizi P, Arlotta A, Scalzone M, Attin G, Ruggiero A, et al. Hepatic veno-occlusive disease. Pediatr Drugs. 2010;12:277–84.
Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–502.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Shulman HM, Hinterberger W. Hepatic veno-occlusive disease–liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant. 1992;10:197–214.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantatation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–68.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9.
Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396 LP–1404.
Lindsey DX, Dx ER, Kim T, Nageshwar P, Nikiforow S, Koreth J, et al. Early clinical predictors of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:137–44.
Lee SH, Yoo KH, Sung KW, Koo HH, Kwon YJ, Kwon MM, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: Incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45:1287–93.
Kammersgaard MB, Kielsen K, Heilmann C, Ifversen M, Müller K. Assessment of the proposed EBMT pediatric criteria for diagnosis and severity grading of sinusoidal obstruction syndrome. Bone Marrow Transplant. 2019;54:1406–18.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaero S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classi fi cation from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2017;53:138–45.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Work. Blood. 1998;92:3599–604.
Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713–20.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. 2019;25:1271–80.
Ouachée-Chardin M, Elie C, de Saint Basile G, Le Deist F, Mahlaoui N, Picard C, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006;117:743–50.
Schulz AS, Classen CF, Mihatsch WA, Guerrini M, Vezzoni P, Frattini A, et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood. 2002;99:3458–60.
Horn B, Reiss U, Matthay K, McMillan A, Cowan M. Veno-occlusive disease of the liver in children with solid tumors undergoing autologous hematopoietic progenitor cell transplantation: a high incidence in patients with neuroblastoma. Bone Marrow Transplant. 2002;29:409–15.
Strouse C, Zhang Y, Zhang MJ, Digilio A, Pasquini M, Horowitz MM, et al. Risk score for the development of veno-occlusive disease after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2018;24:2072–80.
Naples JC, Skeens MA, Auletta J, Rangarajan H, Abu-Arja R, Horowitz E, et al. Anicteric veno-occlusive disease after hematopoietic stem cell transplantation in children. Bone Marrow Transplant. 2016;51:135–7.
Myers KC, Dandoy C, El-Bietar J, Davies SM, Jodele S. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:379–81.
Carreras E, Rosiñol L, Terol MJ, Alegre A, de Arriba F, García-Laraña J, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13:1448–54.
Akil A, Zhang Q, Mumaw CL, Raiker N, Yu J, Velez de Mendizabal N, et al. Biomarkers for diagnosis and prognosis of sinusoidal obstruction syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1739–45.
Lassau N, Auperin A, Leclere J, Bennaceur A, Valteau-Couanet D, Hartmann O. Prognostic value of Doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation. 2002;74:60–6.
Corbacioglu S, Richardson PG. Defibrotide for children and adults with hepatic veno-occlusive disease post hematopoietic cell transplantation. Expert Rev Gastroenterol Hepatol. 2017;11:885–98.
Mohty M, Malard F, Abecasis M, Aerts E, Alaskar AS, Aljurf M, et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group. Bone Marrow Transplant. 2019;55:485–95.
Szmit Z, Kałwak K, Mielcarek-Siedziuk M, Ussowicz M, Owoc-Lempach J, Gorczyńska E. Veno-occlusive disease in children and adolescents after hematopoietic stem cell transplantation: Did the Modified Seattle Criteria fit the characteristics of pediatric population? Adv Clin Exp Med. 2020;29:339–44.
Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D, et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant. 2004;33:189–95.
Bajwa RPS, Mahadeo KM, Taragin BH, Dvorak CC, MacArthur J, Jeyapalan A, et al. Consensus Report by Pediatric Acute Lung Injury and Sepsis Investigators and Pediatric Blood and Marrow Transplantation Consortium Joint Working Committees: supportive care guidelines for management of veno-occlusive disease in children and adolescents. Biol Blood Marrow Transplant. 2017;23:1817–25.
Maximova N, Ferrara G, Minute M, Pizzel A, Kiren V, Montico M, et al. Experience from a single paediatric transplant centre with identification of some protective and risk factors concerning the development of hepatic veno-occlusive disease in children after allogeneic hematopoietic stem cell transplant. Int J Hematol. 2014;99:766–72.
Faraci M, Bertaina A, Luksch R, Calore E, Lanino E, Saglio F, et al. Sinusoidal obstruction syndrome/veno-occlusive disease after autologous or allogeneic hematopoietic stem cell transplantation in children: a retrospective study of the Italian Hematology-Oncology Association À Hematopoietic Stem Cell Transplantation Group. Biol Blood Marrow Transplant. 2019;25:313–20.
Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181:816–27.
Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: Diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–57.
Rio B, Andreu G, Zittoun R, Marrow B. Thrombocytopenia in venocclusive disease after bone marrow transplantation or chemotherapy. Blood. 1986;6:1773–6.
Toh HC, McAfee SL, Sackstein R, Cox BF, Colby C, Spitzer TR. Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant. 1999;24:891–5.
Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–60.
Norol F, Kuentz M, Cordonnier C, Beaujean F, Haioun C, Vernant JP, et al. Influence of clinical status on the efficiency of stored platelet transfusion. Br J Haematol. 1994;86:125–9.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljruf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:1–7.
Rajagopal R, Phillips MB, Gottardo NG. “Pre-emptive strike”— the case for early treatment of hepatic sinusoidal obstruction syndrome with defibrotide. Pediatric Blood and Cancer. 2018;1:10–3.
Grupp SA, Richardson PG, Smith AR, Kernan NA, Antin JH, Lehmann L, et al. Timing of initiation of defibrotide (DF) post-diagnosis of hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after hematopoietic stem cell transplantation (HSCT): Final data from an expanded-access protocol. Journal of Clinical Oncology. 2017;35:7047.
Richardson P, Aggarwal S, Topaloglu O, Villa KF, Corbacioglu S. Systematic review of defibrotide studies in the treatment of veno- occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transpl. 2019;54:1951–62.
Dalle J, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and strati fi cation, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016;22:400–9.
Strouse C, Richardson P, Prentice G, Korman S, Hume R, Nejadnik B, et al. De fi brotide for treatment of severe veno-occlusive disease in pediatrics and adults: an exploratory analysis using data from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2016;22:1306–12.
Richardson PG, Smith AR, Triplett BM, Kernan NA, Groupp SA, Antin JH, et al. Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation. Br J Haematol. 2017;178:112–8.
Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168:481–91.
Cao Z, Villa KF, Lipkin CB, Robinson SB, Nejadnik B, Dvorak CC. Burden of illness associated with sinusoidal obstruction syndrome/veno-occlusive disease in patients with hematopoietic stem cell transplantation. J Med Econ. 2017;20:871–83.
Acknowledgements
Authors express their deep appreciation for prof. Selim Corbacioglu for a long-lasting cooperation and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szmit, Z., Gorczynska, E., Król, A. et al. Introduction of new pediatric EBMT criteria for VOD diagnosis: is it time-saving or money-wasting?. Bone Marrow Transplant 55, 2138–2146 (2020). https://doi.org/10.1038/s41409-020-0918-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0918-1
This article is cited by
-
Acute kidney injury and risk factors in pediatric patients undergoing hematopoietic stem cell transplantation
Pediatric Nephrology (2024)
-
Prevention of veno-occlusive disease/sinusoidal obstruction syndrome: a never-ending story and no easy answer
Bone Marrow Transplantation (2023)
-
Identification of an asymptomatic Shwachman–Bodian–Diamond syndrome mutation in a patient with acute myeloid leukemia
International Journal of Hematology (2022)
-
Acute kidney injury in pediatric hematopoietic cell transplantation: critical appraisal and consensus
Pediatric Nephrology (2022)
-
The coming of age of the pediatric EBMT criteria
Bone Marrow Transplantation (2021)