Abstract
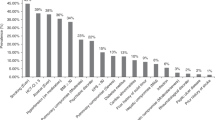
Care of long-term survivors of allogeneic transplant is known to be variable despite international guidelines and accreditation standards. In 2014 a survey of UK NHS-based adult transplant centres identified significant barriers to delivery of long-term follow-up services. In 2019, we repeated the survey to assess changes over a 5-year period when health service policies had mandated JACIE accreditation incorporating standards for long-term care. Improvements were seen in the number of centres having a dedicated long-term follow-up clinic for allogeneic transplant recipients (52% versus 33%) and a standard operating procedure (88% versus 69%). Inclusion of psychological support in standard operating procedures remained low at both time points (32% versus 28%). There was ongoing variation in practice regarding vaccination programmes, access to cancer screening, and audit processes between centres. Perceived barriers to implementation of comprehensive long-term follow-up clinics were similar in 2019; mainly resourcing clinical staff and psychological support. Whilst the survey reflects the changing practice of transplant centres, best explained by increasing recognition of late effects and survivorship by clinicians, health service policy and JACIE accreditation standards, further developments are warranted to address unmet healthcare needs of long-term HSCT survivors, especially access to psychological support, cancer screening and vaccinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–71.
Hamblin A, Greenfield D, Gilleece M, Salooja N, Kenyon M, Morris E, et al. Provision of long-term monitoring and late effects services following adult allogeneic haematopoietic stem cell transplant: a survey of UK NHS-based programmes. Bone Marrow Transplant. 2017;52:889–94.
Hashmi S, Lee S, Savani B, Burns L, Wingard J, Perales M, et al. ASBMT practice guidelines committee survey on long-term follow-up clinics for hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2018;24:1119–24.
PDE Miller, de Silva T, Skinner R, Gilleece M, Peniket A, Hamblin A, et al. Routine vaccination practice after adult and paediatric allogeneic haematopoietic stem cell transplant: a survey of UK NHS programmes. Bone Marrow Transplant. 2017;52:775–7.
Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Infectious Diseases Working Party EBMT. Bone Marrow Transplant. 2020;55:126–36.
NHS England (version B04/P/a) Jan 2013. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/01/b04-haematp-stem-cll-transplt.pdf. Accessed 14 Oct 2020.
Snowden JA, McGrath E, Duarte RF, Saccardi R, Orchard K, Worel N, et al. JACIE accreditation for blood and marrow transplantation: past, present and future directions of an international model for healthcare quality improvement. Bone Marrow Transplant. 2017;52:1367–71.
Saccardi R, McGrath E, Snowden AJ. JACIE Accreditation of HSCT Programs. The EBMT handbook. 2019:35–40.
Snowden JA, Saccardi R, Orchard K, Ljungman P, Duarte RF, Labopin M, et al. Benchmarking of survival outcomes following haematopoietic stem cell transplantation: a review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. 2020;55:681–94.
BSBMTCT Executive summary of transplant and cellular therapy outcomes in UK/ROI. 10th ed. (unpublished data).
Public Health England with NHS England and NHS Improvement Public Health Commissioning. NHS Public Health Functions Agreement 2019–20. NHS Public Health Functions 2019–20 Service Specification No. 24 NHS Breast Screening Programme Jul 2019. https://www.england.nhs.uk/wp-content/uploads/2017/04/Service-Specification-No.24-Breast_Screening_Programme.pdf. Accessed 14 Oct 2020.
Public Health Scotland 2020. https://www.isdscotland.org/Health-Topics/Cancer/Breast-Screening/. Accessed 14 Oct 2020.
Public Health Wales 2020. http://www.breasttestwales.wales.nhs.uk/home. Accessed 14 Oct 2020.
Health and Social Care Public Health Agency Overview of Northern Ireland Breast Screening Programme 2020. http://www.cancerscreening.hscni.net/Overview_of_Breast_Screening_Programme.htm. Accessed 14 Oct 2020.
Public Health England with NHS England and NHS Improvement Public Health Commissioning. NHS Public Health Functions Agreement 2019-20. Service Specification No. 25 NHS Cervical Screening Programme. July 2019. https://www.england.nhs.uk/wp-content/uploads/2017/04/Service-Specification-No.25-Cervical_Screening.pdf. Accessed 14 Oct 2020.
Public Health Scotand 2020. https://www.isdscotland.org/Health-Topics/Cancer/Cervical-Screening/. Accessed 14 Oct 2020.
Public Health Wales 2020. http://www.cervicalscreeningwales.wales.nhs.uk/. Accessed 14 Oct 2020.
Health and Social Care Public Health Agency Overview of Northern Ireland Cervical Screening Programme 2020. https://www.publichealth.hscni.net/directorate-public-health/service-development-and-screening/cervical-cancer-screening. Accessed 14 Oct 2020.
Public Health England. Protocols for Surveillance of Women at Very High Risk of Developing Breast Cancer Oct 2020. https://www.gov.uk/government/publications/breast-screening-higher-risk-women-surveillance-protocols/protocols-for-surveillance-of-women-at-higher-risk-of-developing-breast-cancer. Accessed 14 Oct 2020.
Acknowledgements
The authors acknowledge the contribution of the data registry team of the British Society of Blood and Marrow Transplantation & Cellular Therapy for their support in providing registry data. We would also like to thank the staff of all the centres who contributed data to this survey.
British Society of Blood and Marrow Transplantation and Cellular Therapy
Fiona L. Dignan1, Julia Lee4, Maria Gilleece6, John A. Snowden8, Kim Orchard9, Deborah Richardson9, Dominic Culligan10, Kavita Raj11, Eduardo Olavarria12, Marie Waller1, Bim Laguda13, Rachael Hough14, Ram Malladi15, Jennifer L. Byrne16, Stephen Robinson17
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
JAS was Chair of NHSE Clinical Reference Group for Blood and Marrow Transplantation from June 2016-June 2020 and declares speaker fees for Sanofi, Jazz, Mallinckrodt, Janssen, Gilead and trial IDMC membership for Kiadis Pharma. FLD is Chair of the NHSE Clinical Reference Group for Blood and Marrow Transplantation from June 2020 and declares speaker fees for Jazz, Mallinckrodt, Pfizer and Janssen. FLD and JAS co-chaired the NHSE Clinical Reference Group for Blood and Marrow Transplantation from April to June 2020. No conflicts of interest were declared by other authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the “British Society of Blood and Marrow Transplantation and Cellular Therapy” are listed below in Acknowledgements.
Supplementary information
Rights and permissions
About this article
Cite this article
Dignan, F.L., Hamblin, A., Chong, A. et al. Survivorship care for allogeneic transplant patients in the UK NHS: changes centre practice, impact of health service policy and JACIE accreditation over 5 years. Bone Marrow Transplant 56, 673–678 (2021). https://doi.org/10.1038/s41409-020-01067-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01067-y
This article is cited by
-
Psychosocial assessment practices for hematopoietic stem cell transplantation: a national survey study
Bone Marrow Transplantation (2023)
-
The lived experience of long-term follow-up clinical care for haematopoietic stem cell recipients in England: a qualitative exploration
Journal of Cancer Survivorship (2023)
-
Managing Survivorship after Hematopoietic Cell Transplantation
Current Hematologic Malignancy Reports (2023)