Abstract
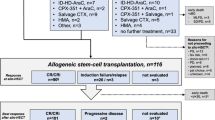
Hypomethylating agents (HMA) for relapsed acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) after allogeneic transplantation (allo-SCT) are most effective when used at the stage of molecular relapse. As Wilm’s Tumor 1 (WT1)- expression has proven to serve as broadly applicable, sensitive and specific minimal residual disease (MRD) marker, we measured WT1-expression in 35 AML and MDS patients using a standardized assay for the guidance of therapy with HMA and donor lymphocyte infusions (DLI). Molecular relapse was detected in median 168 days post-transplant prompting therapy with a median of six HMA cycles and at least one DLI (n = 22, 63%). Hereby, 13 patients (37%) achieved major response (=MRD− complete remission [CR]), and 7 patients (20%) achieved minor response (=MRD+ CR), whereas 15 patients (43%) progressed into hematologic relapse. Two-year overall survival (OS) rate was 35% including 11 patients (31%) with ongoing MRD− remission for a median of 21 months. Patients with the major response after six cycles had significantly better OS suggesting that those not achieving MRD negativity after six cycles are candidates for alternative therapies. Combining MRD-monitoring of WT1-expression and preemptive therapy with HMA and DLI appears as a practicable and efficient approach for imminent relapse after allo-SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica. 2018;103:237–45.
Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–606.
Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–45.
Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–9.
Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27:1229–35.
Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions-a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transpl. 2015;21:653–60.
Schroeder T, Rautenberg C, Krüger W, Platzbecker U, Bug G, Steinmann J, et al. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann Hematol. 2018;97:335–42.
Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2016;101:879–83.
Miyamoto T, Fukuda T, Nakashima M, Henzan T, Kusakabe S, Kobayashi N, et al. Donor lymphocyte infusion for relapsed hematological malignancies after unrelated allogeneic bone marrow transplantation facilitated by the Japan Marrow Donor Program. Biol Blood Marrow Transpl. 2017;23:938–44.
Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20:228.
Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transpl. 2016;51:1431–8.
Bornhauser M, Thiede C. Chimerism analysis after allogeneic stem cell transplantation. Haematologica. 2005;90:1301A–1301A.
Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94:1613–7.
Wang Y, Wu D-P, Liu Q-F, Qin Y-Z, Wang J-B, Xu L-P, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124:1880–6.
Tang F-F, Xu L-P, Zhang X-H, Chen H, Chen Y-H, Han W, et al. Monitoring of post-transplant CBFB-MYH11 as minimal residual disease, rather than KIT mutations, can predict relapse after allogeneic haematopoietic cell transplantation in adults with inv(16) acute myeloid leukaemia. Br J Haematol. 2018;180:448–51.
Zhou Y, Othus M, Walter RB, Estey EH, Wu D, Wood BL. Deep NPM1 sequencing following allogeneic hematopoietic cell transplantation improves risk assessment in adults with NPM1-mutated AML. Biol Blood Marrow Transpl. 2018;24:1615–20.
Bacher U, Badbaran A, Fehse B, Zabelina T, Zander AR, Kröger N. Quantitative monitoring of NPM1 mutations provides a valid minimal residual disease parameter following allogeneic stem cell transplantation. Exp Hematol. 2009;37:135–42.
Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013;122:83–92.
Lange T, Hubmann M, Burkhardt R, Franke G-N, Cross M, Scholz M, et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia. 2011;25:498–505.
Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. 2006;20:1217–20.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–201.
Rautenberg C, Pechtel S, Hildebrandt B, Betz B, Dienst A, Nachtkamp K, et al. Wilms’ tumor 1 gene expression using a Standardized European LeukemiaNet-certified assay compared to other methods for detection of minimal residual disease in myelodysplastic syndrome and acute myelogenous leukemia after allogeneic blood stem cell transplantation. Biol Blood Marrow Transpl. 2018;24:2337–43.
Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19:1668–79.
Claiborne J, Bandyopathyay D, Roberts C, Hawks K, Aziz M, Simmons G, et al. Managing post allograft relapse of myeloid neoplasms: azacitidine and donor lymphocyte infusions as salvage therapy. Leuk Lymphoma. 2019;60:2733–43.
Schuler E, Boughoufala S, Rautenberg C, Nachtkamp K, Dienst A, Fenk R, et al. Relapse patterns and treatment strategies in patients receiving allogeneic hematopoietic stem cell transplantation for myeloid malignancies. Ann Hematol. 2019;98:1225–35.
Candoni A, Tiribelli M, Toffoletti E, Cilloni D, Chiarvesio A, Michelutti A, et al. Quantitative assessment of WT1 gene expression after allogeneic stem cell transplantation is a useful tool for monitoring minimal residual disease in acute myeloid leukemia. Eur J Haematol. 2009;82:61–8.
Yoon J-H, Jeon Y-W, Yahng S-A, Shin S-H, Lee S-E, Cho B-S, et al. Wilms tumor gene 1 expression as a predictive marker for relapse and survival after hematopoietic stem cell transplantation for myelodysplastic syndromes. Biol Blood Marrow Transpl. 2015;21:460–7.
Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JHF, et al. NCI First International Workshop on the Biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on treatment of relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1467–503.
Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination lenalidomide and azacitidine: a novel salvage therapy in patients who relapse after allogeneic stem-cell transplantation for acute myeloid leukemia. J Clin Oncol. 2019;37:580–8.
Schroeder TM, Rautenberg C, Christopeit M, Stelljes M, Schmidt E, Groth C, et al. Treatment of MDS, AML and CMML relapse after allogeneic blood stem cell transplantation with azacitidine, lenalidomide and donor lymphocyte infusions results from the Second Interim Analysis of the Prospective Azalena-Trial (NCT02472691). Blood. 2018;132:703.
Schuler E, Wagner-Drouet E-M, Ajib S, Bug G, Crysandt M, Dressler S, et al. Treatment of relapse after allogeneic hematopoietic stem cell transplantation with venetoclax, hypomethylating agents and DLI—a Retrospective Multi Center Study. Blood. 2019;134:4563.
Rautenberg C, Germing U, Pechtel S, Lamers M, Fischermanns C, Jäger P, et al. Prognostic impact of peripheral blood WT1- mRNA expression in patients with MDS. Blood Cancer J. 2019;9:1–8.
Duncavage EJ, Jacoby MA, Chang GS, Miller CA, Edwin N, Shao J, et al. Mutation clearance after transplantation for myelodysplastic syndrome. N. Engl J Med. 2018;379:1028–41.
Brambati C, Galbiati S, Xue E, Toffalori C, Crucitti L, Greco R, et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101:e157–61.
Sockel K, Wermke M, Radke J, Kiani A, Schaich M, Bornhäuser M, et al. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica. 2011;96:1568–70.
Waterhouse M, Pfeifer D, Duque-Afonso J, Follo M, Duyster J, Depner M, et al. Droplet digital PCR for the simultaneous analysis of minimal residual disease and hematopoietic chimerism after allogeneic cell transplantation. Clin Chem Lab Med. 2019;57:641–7.
Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25:1064–72.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25.
Acknowledgements
We thank the staff of the Transplantation Unit of the Department of Hematology, Oncology, and Clinical Immunology for excellent patient care.
Author information
Authors and Affiliations
Contributions
Study conception and design: CR, GK, and TS. Collection and assembly of data: CR, AB, SP, CF, and TS. Data analysis and interpretation: CR, AB, and TS. Manuscript writing: CR and TS. Final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
CR received financial travel support from Celgene Deutschland GmbH. TS received financial travel support, lecture fees, research funding, and participated in advisory boards for Celgene GmbH. TS received financial travel support, lecture fees, and participated in advisory boards for Janssen-Cilag GmbH. A Rotorgene PCR cycler was provided free of charge by Qiagen (Hilden, Germany) for routine use in this study. No other financial or logistic support was provided by the company for this analysis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rautenberg, C., Bergmann, A., Pechtel, S. et al. Wilm’s Tumor 1-guided preemptive treatment with hypomethylating agents for molecular relapse of AML and MDS after allogeneic transplantation. Bone Marrow Transplant 56, 442–450 (2021). https://doi.org/10.1038/s41409-020-01039-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01039-2