Abstract
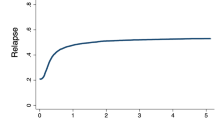
Aggressive NK-cell leukemia (ANKL) has a fulminant clinical course with a poor prognosis. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently the only curative treatment. Using the Japanese transplant registry data, the outcomes of 59 ANKL patients who underwent first allo-HSCT were analyzed. Twenty-nine patients received stem cells from cord blood (CB), 18 from peripheral blood, and 12 from bone marrow. At the time of transplant 21 patients had complete response (CR), and 7 partial response (PR), but 31 without response. The 1-year and 5-year overall survival (OS) were 33.9% and 27.3%, respectively. The 1-year cumulative incidences of relapse or progression was 55.5%, and that of non-relapse mortality was 12.1%. The OS was significantly better for patients with CR or PR at the time of allo-HSCT (P = 0.046), which was equivalent to that for patients who experienced primary induction failure at the time of allo-HSCT but achieved CR afterwards (40.6% versus 32.0% at 5 years; P = 0.95). Patients receiving CB had a significantly better OS than those receiving stem cells from others (37.3% versus 16.2% at 5 years; P = 0.04). Patients achieving event-free survival at 12 months after allo-HSCT had good outcomes with 5-year OS of 85.2%.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Loughran TP Jr., Kadin ME, Starkebaum G, Abkowitz JL, Clark EA, Disteche C, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann Intern Med. 1985;102:169–75. https://doi.org/10.7326/0003-4819-102-2-169.
Fernandez LA, Pope B, Lee C, Zayed E. Aggressive natural killer cell leukemia in an adult with establishment of an NK cell line. Blood. 1986;67:925–30.
Koizumi S, Seki H, Tachinami T, Taniguchi M, Matsuda A, Taga K, et al. Malignant clonal expansion of large granular lymphocytes with a Leu-11+, Leu-7- surface phenotype: in vitro responsiveness of malignant cells to recombinant human interleukin 2. Blood. 1986;68:1065–73.
Jaffe ESHN, Stein H, Vardiman JW. Pathology and Genetics of Tumours of Haematopoietic and lymphoid Tissues (IARC, 2001).
Oshimi K. Leukemia and lymphoma of natural killer lineage cells. Int J Hematol. 2003;78:18–23. https://doi.org/10.1007/bf02983235.
Imamura N, Kusunoki Y, Kawa-Ha K, Yumura K, Hara J, Oda K, et al. Aggressive natural killer cell leukaemia/lymphoma: report of four cases and review of the literature. Possible existence of a new clinical entity originating from the third lineage of lymphoid cells. Br J Haematol. 1990;75:49–59. https://doi.org/10.1111/j.1365-2141.1990.tb02615.x.
Kawa-Ha K, Ishihara S, Ninomiya T, Yumura-Yagi K, Hara J, Murayama F, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Investig. 1989;84:51–55. https://doi.org/10.1172/jci114168.
Loughran TP Jr. Clonal diseases of large granular lymphocytes. Blood. 1993;82:1–14.
Yamamoto T, Iwasaki T, Watanabe N, Oshimi K, Naito M, Tsuruo T, et al. Expression of multidrug resistance P-glycoprotein on peripheral blood mononuclear cells of patients with granular lymphocyte-proliferative disorders. Blood. 1993;81:1342–6.
Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93:599–606.
Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18:763–70. https://doi.org/10.1038/sj.leu.2403262.
Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002;87:1343–5.
Ishida F, Ko YH, Kim WS, Suzumiya J, Isobe Y, Oshimi K, et al. Aggressive natural killer cell leukemia: therapeutic potential of L-asparaginase and allogeneic hematopoietic stem cell transplantation. Cancer Sci. 2012;103:1079–83. https://doi.org/10.1111/j.1349-7006.2012.02251.x.
Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. J Hematol Oncol. 2016;9:41. https://doi.org/10.1186/s13045-016-0271-4.
Teshima T, Miyaji R, Fukuda M, Ohshima K. Bone-marrow transplantation for Epstein-Barr-virus-associated natural killer cell-large granular lymphocyte leukaemia. Lancet. 1996;347:1124. https://doi.org/10.1016/s0140-6736(96)90325-6.
Murashige N, Kami M, Kishi Y, Kim SW, Takeuchi M, Matsue K, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. Br J Haematol. 2005;130:561–7. https://doi.org/10.1111/j.1365-2141.2005.05651.x.
Takami A, Nakao S, Yachie A, Kasahara Y, Okumura H, Miura Y, et al. Successful treatment of Epstein-Barr virus-associated natural killer cell large granular lymphocytic leukaemia using allogeneic peripheral blood stem cell transplantation. Bone Marrow Transpl. 1998;21:1279–82. https://doi.org/10.1038/sj.bmt.1701262.
Ito T, Makishima H, Nakazawa H, Kobayashi H, Shimodaira S, Nakazawa Y, et al. Promising approach for aggressive NK cell leukaemia with allogeneic haematopoietic cell transplantation. Eur J Haematol. 2008;81:107–11. https://doi.org/10.1111/j.1600-0609.2008.01090.x.
Tang YT, Wang D, Luo H, Xiao M, Zhou HS, Liu D, et al. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J. 2017;7:660. https://doi.org/10.1038/s41408-017-0021-z.
Ishida F. Aggressive NK-CellLeukemia. Front Pediatrics. 2018;6:292. https://doi.org/10.3389/fped.2018.00292.
Hamadani M, Kanate AS, DiGilio A, Ahn KW, Smith SM, Lee JW, et al. Allogeneic hematopoietic cell transplantation for aggressive NK cell leukemia. A Center for International Blood and Marrow Transplant Research Analysis. Biol Blood Marrow Transpl. 2017;23:853–6. https://doi.org/10.1016/j.bbmt.2017.01.082.
Jeong SH, Song HN, Park JS, Yang DH, Koh Y, Yoon SS, et al. Allogeneic stem cell transplantation for patients with natural killer/T cell lymphoid malignancy: a multicenter analysis comparing upfront and salvage transplantation. Biol Blood Marrow Transpl. 2018;24:2471–8. https://doi.org/10.1016/j.bbmt.2018.07.034.
Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes, chromosomes cancer. 2005;44:247–55. https://doi.org/10.1002/gcc.20245.
Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H. et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010;21:1032–40. https://doi.org/10.1093/annonc/mdp418.
Dufva O, Kankainen M, Kelkka T, Sekiguchi N, Awad SA, Eldfors S, et al. Aggressive natural killer-cell leukemia mutational landscape and drug profiling highlight JAK-STAT signaling as therapeutic target. Nat Commun. 2018;9:1567. https://doi.org/10.1038/s41467-018-03987-2.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74. https://doi.org/10.1532/ijh97.06239.
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10. https://doi.org/10.1007/s12185-015-1894-x.
Fujimoto A, Hiramoto N, Yamasaki S, Inamoto Y, Uchida N, Maeda T, et al. Risk factors and predictive scoring system for post-transplant lymphoproliferative disorder after hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2019;25:1441–9. https://doi.org/10.1016/j.bbmt.2019.02.016.
Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–73. https://doi.org/10.1200/jco.2013.51.5866.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transpl. 2001;28:1001–11. https://doi.org/10.1038/sj.bmt.1703271.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2009;15:367–9. https://doi.org/10.1016/j.bbmt.2008.12.497.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. https://doi.org/10.1200/jco.1999.17.4.1244.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. https://doi.org/10.1200/jco.2006.09.2403.
Maurer MJ, Bachy E, Ghesquieres H, Ansell SM, Nowakowski GS, Thompson CA, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096–101. https://doi.org/10.1002/ajh.24492.
Urbano-Ispizua A, Pavletic SZ, Flowers ME, Klein JP, Zhang MJ, Carreras J, et al. The impact of graft-versus-host disease on the relapse rate in patients with lymphoma depends on the histological subtype and the intensity of the conditioning regimen. Biol Blood Marrow Transpl. 2015;21:1746–53. https://doi.org/10.1016/j.bbmt.2015.05.010.
Acknowledgements
We thank all the physicians and data managers at the centers who contributed to gathering data on transplantation for the Transplant Registry Unified Management Program and all the members of the Data Management Committees of Japan Society for Hematopoietic Cell Transplantation. This work was supported in part by the Practical Research Project for Allergic Disease and Immunology (Research Technology of Medical Transplantation), Japan Agency for Medical Research and Development, AMED.
Author information
Authors and Affiliations
Contributions
A.F. and R.S. designed the study. A.F. performed analyses and wrote the manuscript. All authors discussed the results, interpreted the data, and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fujimoto, A., Ishida, F., Izutsu, K. et al. Allogeneic stem cell transplantation for patients with aggressive NK-cell leukemia. Bone Marrow Transplant 56, 347–356 (2021). https://doi.org/10.1038/s41409-020-01009-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01009-8
This article is cited by
-
Possible Concomitant Aggressive NK Cell Leukemia and EBV-positive T-cell lymphoma; Using the online beta version of WHO-HAEM5 and videoconferencing software to make diagnoses accessible in an emerging economy
Diagnostic Pathology (2023)
-
Reconstitution of Natural Killer cells after allogeneic hematopoietic stem cell transplantation is facilitated by Huiyang-Guben decoction through activating the Smad7/Stat3 signal pathway
Molecular and Cellular Biochemistry (2023)
-
Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review
Stem Cell Research & Therapy (2022)