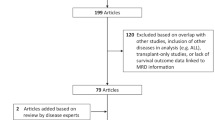
Abstract
Detectable measurable residual disease (MRD) is a key prognostic factor in both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) patients. Thus, we conducted a survey in EBMT transplant centers focusing on pre- and post-allo-HCT MRD. One hundred and six centers from 29 countries responded. One hundred had a formal strategy for routine MRD assessment, 91 for both ALL and AML. For ALL (n = 95), assessing MRD has been routine practice starting from 2010 (range, 1990–2019). Techniques used for MRD assessment consisted of PCR techniques alone (n = 27), multiparameter flow cytometry (MFC, n = 16), both techniques (n = 43), next-generation sequencing (NGS) + PCR (n = 2), or PCR + MFC + NGS (n = 7). The majority of centers assessed MRD every 2–3 months for 2 (range, 1-until relapse) years. For AML, assessing MRD was routine in 92 centers starting in 2010 (range 1990–2019). Assessment of MRD was by PCR (n = 23), MFC (n = 13), both PCR and MFC (n = 39), both PCR and NGS (n = 3), and by all three techniques (n = 14). The majority assesses MRD for AML every 2–3 months for 2 (range, 1-until relapse) years. This survey is the first step in the aim to include MRD status as a routine registry capture parameter in acute leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Della Starza I, Chiaretti S, De Propris MS, Elia L, Cavalli M, De Novi LA. et al. Minimal residual disease in acute lymphoblastic leukemia: technical and clinical advances. Front Oncol. 2019;9:726. https://doi.org/10.3389/fonc.2019.00726.
Maffini E, Saraceni F, Lanza F. Treatment of adult patients with relapsed/refractory B-cell Philadelphia-negative acute lymphoblastic leukemia. Clin Hematol Int. 2019;1:85–93. https://doi.org/10.2991/chi.d.190503.002.
Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3:e170580. https://doi.org/10.1001/jamaoncol.2017.0580.
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol: Off J Am Soc Clin Oncol. 2018;36:1486–97. https://doi.org/10.1200/JCO.2017.76.3425.
Kronke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:2709–16. https://doi.org/10.1200/JCO.2011.35.0371.
Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association Group. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35:185–93. https://doi.org/10.1200/JCO.2016.67.1875.
Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134:935–45. https://doi.org/10.1182/blood.2018886960.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. https://doi.org/10.1182/blood-2016-08-733196.
Baron F, Efficace F, Cannella L, Willemze R, Vignetti M, Muus P. et al. Long-term follow-up of a trial comparing post-remission treatment with autologous or allogeneic bone marrow transplantation or intensive chemotherapy in younger acute myeloid leukemia patients. Haematologica. 2019. https://doi.org/10.3324/haematol.2019.221333.
Baron F, Efficace F, Cannella L, Muus P, Trisolini S, Halkes CJM, et al. Impact of the type of anthracycline and of stem cell transplantation in younger patients with acute myeloid leukaemia: long-term follow up of a phase III study. Am J Hematol. 2020;95:749–58. https://doi.org/10.1002/ajh.25795.
Cloos J, Ossenkoppele GJ, Dillon R. Minimal residual disease and stem cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2019;2019:617–625. https://doi.org/10.1182/hematology.2019000006.
Gandemer V, Pochon C, Oger E, Dalle JH, Michel G, Schmitt C, et al. Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol. 2014;165:392–401. https://doi.org/10.1111/bjh.12749.
Oran B, Jorgensen JL, Marin D, Wang S, Ahmed S, Alousi AM, et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica. 2017;102:110–7. https://doi.org/10.3324/haematol.2016.144253.
Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–21. https://doi.org/10.1182/blood-2013-06-506725.
Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102:865–73. https://doi.org/10.3324/haematol.2016.159343.
Baron F, Stevens-Kroef M, Kicinski M, Meloni G, Muus P, Marie JP, et al. Impact of induction regimen and of allogeneic hematopoietic cell transplantation on outcome in younger adults patients with acute myeloid leukemia with a monosomal karyotype. Haematologica. 2019;104:1168–75. https://doi.org/10.3324/haematol.2018.204826.
Morsink LM, Othus M, Bezerra ED, Wood BL, Fang M, Sandmaier BM. et al. Impactof pretransplant measurable residual disease on the outcome of allogeneichematopoietic cell transplantation in adult monosomal karyotype AML. Leukemia. 2020. https://doi.org/10.1038/s41375-020-0717-0.
Czyz A, Nagler A. The role of measurable residual disease (MRD) in hematopoietic stem cell transplantation for hematological malignancies focusing on acute leukemia. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20215362.
Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socie G, Ljungman P, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: a registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am J Hematol. 2018;93:1142–52. https://doi.org/10.1002/ajh.25211.
Canaani J, Labopin M, Huang XJ, Ciceri F, Van Lint MT, Bruno B, et al. Minimal residual disease status predicts outcome of acute myeloid leukaemia patients undergoing T-cell replete haploidentical transplantation. An analysis from the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2018. https://doi.org/10.1111/bjh.15540.
Pavlu J, Labopin M, Niittyvuopio R, Socie G, Yakoub-Agha I, Wu D, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12:108. https://doi.org/10.1186/s13045-019-0790-x.
Nagler A, Dholaria B, Labopin M, Socie G, Huynh A, Itala-Remes M, et al. The impact of anti-thymocyte globulin on the outcomes of Patients with AML with or without measurable residual disease at the time of allogeneic hematopoietic cell transplantation. Leukemia. 2019. https://doi.org/10.1038/s41375-019-0631-5.
Baron F, Labopin M, Ruggeri A, Sierra J, Robinson S, Labussiere-Wallet H, et al. Impact of detectable measurable residual disease on umbilical cord blood transplantation. Am J Hematol. 2020. https://doi.org/10.1002/ajh.25879.
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30:1456–64. https://doi.org/10.1038/leu.2016.46.
Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33:1275–84. https://doi.org/10.1200/JCO.2014.58.4631.
Wethmar K, Matern S, Esseling E, Angenendt L, Pfeifer H, Bruggemann M, et al. Monitoring minimal residual/relapsing disease after allogeneic haematopoietic stem cell transplantation in adult patients with acute lymphoblastic leukaemia. Bone Marrow Transplant. 2020. https://doi.org/10.1038/s41409-020-0801-0.
Pozzi S, Geroldi S, Tedone E, Luchetti S, Grasso R, Colombo N, et al. Leukaemia relapse after allogeneic transplants for acute myeloid leukaemia: predictive role of WT1 expression. Br J Haematol. 2013;160:503–9. https://doi.org/10.1111/bjh.12181.
Voso MT, Ottone T, Lavorgna S, Venditti A, Maurillo L, Lo-Coco F, et al. MRD in AML: the role of new. Tech Front Oncol. 2019;9:655. https://doi.org/10.3389/fonc.2019.00655.
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91. https://doi.org/10.1182/blood-2017-09-801498.
Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132:1703–13. https://doi.org/10.1182/blood-2018-02-829911.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic. Cell Transplant Clin Hematol Int. 2020;2:2–4. https://doi.org/10.2991/chi.d.191207.001.
Theunissen P, Mejstrikova E, Sedek L, van der Sluijs-Gelling AJ, Gaipa G, Bartels M, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2017;129:347–57. https://doi.org/10.1182/blood-2016-07-726307.
Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol. 2019;94:902–12. https://doi.org/10.1002/ajh.25514.
Bazarbachi A, Labopin M, Battipaglia G, Djabali A, Forcade E, Arcese W, et al. Allogeneic stem cell transplantation for FLT3-mutated acute myeloid leukemia: in vivo T-cell depletion and posttransplant sorafenib maintenance improve survival. A Retrospective Acute Leukemia Working Party-European Society for Blood and Marrow Transplant Study. Clin Hematol Int. 2019;1:58–74. https://doi.org/10.2991/chi.d.190310.001.
Culos K, Byrne M. Salvage therapy after allogeneic hematopoietic cell transplantation: targeted and low-intensity treatment options in myelodysplastic syndrome and acute myeloid leukemia. Clin Hematol Int. 2019;1:94–100. https://doi.org/10.2991/chi.d.190503.001.
Ehx G, Fransolet G, de Leval L, D’Hondt S, Lucas S, Hannon M, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2017;6:e1314425. https://doi.org/10.1080/2162402X.2017.1314425.
Lee CJ, Savani BN, Mohty M, Gorin NC, Labopin M, Ruggeri A, et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2019;54:519–30. https://doi.org/10.1038/s41409-018-0286-2.
Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol. 2019;184:782–7. https://doi.org/10.1111/bjh.15691.
Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019;54:1815–26. https://doi.org/10.1038/s41409-019-0536-y.
Funding
FB is Senior Research Associate at the National Fund for Scientific Research (FNRS) Belgium.
Author information
Authors and Affiliations
Contributions
AN wrote the manuscript, designed the study, and interpreted the data; FB wrote the manuscript and interpreted the data; ML designed the study, analyzed and interpreted the data, and edited the manuscript; EP designed the study, analyzed and interpreted the data, and edited the manuscript; MM designed the study, interpreted the data, and edited the manuscript; all remaining authors helped in the design of the study, interpreted the data, and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
FB has received travel grants from Celgene, Abbvie, Novartis, and Sanofi as well as honoraria from Merck and Abbvie. The remaining authors declare that they have no relevant conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nagler, A., Baron, F., Labopin, M. et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: a survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant 56, 218–224 (2021). https://doi.org/10.1038/s41409-020-01005-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01005-y
This article is cited by
-
Autologous stem cell transplantation in adult patients with intermediate-risk acute myeloid leukemia in first complete remission and no detectable minimal residual disease. A comparative retrospective study with haploidentical transplants of the global committee and the ALWP of the EBMT
Bone Marrow Transplantation (2023)
-
Post-transplant maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation harmonizing multiple therapeutic modalities including targeted therapy, immunotherapy and cellular therapy
International Journal of Hematology (2023)
-
Fludarabine/TBI 8 Gy versus fludarabine/treosulfan conditioning in patients with AML in first complete remission: a study from the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Impact of disease burden on clinical outcomes of AML patients receiving allogeneic hematopoietic cell transplantation: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2023)
-
Matched related versus unrelated versus haploidentical donors for allogeneic transplantation in AML patients achieving first complete remission after two induction courses: a study from the ALWP/EBMT
Bone Marrow Transplantation (2023)