Abstract
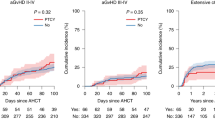
Posttransplant high-dose cyclophosphamide (PTCy) effectively prevents GvHD after haploidentical SCT. However, its use in HLA-matched SCT has been less explored. Fifty-six consecutive patients who underwent allo-SCT for hematological malignancies have been included in this prospective single-center protocol. Donors have been HLA-identical siblings, fully-matched unrelated or 1-allele-mismatched unrelated donors in 30%, 32%, and 37% of cases, respectively. Nine patients have received a TBI-containing MAC regimen, while the remaining (84%) received RIC platforms based on Fludarabine plus Busulfan/Melphalan. Due to the high graft failure (GF) rate (21%) in a preliminary analysis in the allo-RIC cohort (n = 29), protocol amendments have been implemented, with no further cases of GF after the introduction of mini-thiotepa (0/18). The overall incidence of grade II–IV acute GvHD is 24% (95% CI: 17–31%) with four steroid-refractory cases. Severe chronic GvHD has occurred in only 1 of 43 evaluable cases. The 1-year NRM and relapse are 18% (95% CI: 12–26%) and 30% (18–42%) and the OS and DFS are 78% and 64%, respectively. These outcomes support the feasibility of using PTCy as a SOC outside the haplo-setting, albeit mini-thiotepa (3 mg/kg) was incorporated in the standard allo-RIC platforms to prevent GF. Despite the limitations of a single-center experience and the short follow-up, these protocols show promising results with particular benefit in reducing the occurrence of moderate-to-severe GvHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing Incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transpl. 2015;21:266–74.
Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ, et al. Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:449–55.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Posttransplant cyclophosphamide for GVHDprophylaxis in HLA matchedsibling or matched-unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Shah MV, Saliba RM, Rondon G, Chen J, Soebbing D, Rus I, et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GVHD prophylaxis for olderpatientsreceiving 10/10 HLA-matchedunrelateddonorhematopoieticstem cell transplantation. Bone Marrow Transpl. 2019;54:601–6.
Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral bloodstem cell transplants. Eur J Haematol. 2019. https://doi.org/10.1111/ejh.13230.
Mukherjee SD, Goffin JR, Taylor V, Anderson KK, Pond GR. Early stopping rules in oncology: considerations for clinicians. Eur J Cancer. 2011;47:2381–6.
Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplasticsyndromes. Myelodysplastic Syndrome subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Blood. 2006;108:836–46.
Parody R, Lopez-Corral L, Godino OL, Cadenas IG, Martinez AP, Vazquez L, et al. GVHD prophylaxis with sirolimus-tacrolimus may overcome the deleterious effect on survival of HLA mismatch after reduced-intensity conditioning allo-SCT. Bone Marrow Transpl. 2015;50:121–6.
Piñana JL, Valcárcel D, Fernández-Avilés F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolatemofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transpl. 2010;45:1449–56.
Martino R, Pérez-Simón JA, Moreno E, Queraltó JM, Caballero D, Mateos M, et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biol Blood Marrow Transpl. 2005;11:437–47.
Kanakry CG, O'Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablativebusulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
García-Cadenas I, Rivera I, Martino R, Esquirol A, Barba P, Novelli S, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transpl. 2017;52:107–13.
Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolaños-Meade J, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high- dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–92.
Mielcarek M, Furlong T, O'Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transpl. 2013;48:537–43.
Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-KadhimiZ, et al. Three prophylaxis regimens (tacrolimus, mycophenolatemofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43.
Patriarca F, Masciulli A, Bacigalupo A, Bregante S, Pavoni C, Finazzi MC, et al. busulfan-or thiotepa-based conditioning in myelofibrosis: a phase II multicenter randomized study from the GITMO group. Biol Blood Marrow Transpl. 2019;25:932–40.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplantat. 2015;50 Suppl 2:S37–9.
Wang Y, Chang YJ, Chen L, Xu LP, Bian ZL, Zhang XH, et al. Low-dose post-transplant cyclophosphamide can mitigate GVHD and enhance the G-CSF/ATG induced GVHD protective activity and improve haploidentical transplant outcomes. Oncoimmunology. 2017;6:e1356152.
Thakar MS, Bonfim C, Walters MC, Storb R, Pasquini R, Burroughs L, et al. Dose-adapted post-transplant cyclophosphamide for HLA-haploidentical transplantation in Fanconianemia. Bone Marrow Transpl. 2017;52:570–3.
Bonfim C, Ribeiro L, Nichele S, Loth G, Bitencourt M, Koliski A, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide for children and adolescents with Fanconi anemia. Biol Blood Marrow Transpl. 2017;23:310–7.
Ayas MF, Al-Seraihi A, Al-Agil A, Al-Ahmari A, Ghemlas I, Ayas M, et al. Successful hematopoietic cell transplantation in Fanconi anemia patients with renal impairment using ultra-reduced doses of cyclophosphamide and fludarabine. Pediatr Blood Cancer. 2018;65:e27371.
Soltermann Y, Heim D, Medinger M, Baldomero H, Halter JP, Gerull S, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98:1485–93.
Klein OR, Chen AR, Gamper C, Loeb D, Zambidis E, Llosa N, et al. Alternative-donor hematopoietic stem cell transplantation with post transplantation cyclophosphamide for nonmalignant disorders. Biol Blood Marrow Transpl. 2016;22:895–901.
Parody R, López-Corral L, Lopez-Godino O, Martinez C, Martino R, Solano C, et al. GvHD prophylaxis with tacrolimus plus sirolimus after reduced intensity conditioning allogeneic transplantation: results of a multicenter study. Bone Marrow Transpl. 2016;51:1524–6.
Piñana JL, Perez-Pitarch A, Garcia-Cadenas I, Barba P, Hernandez-Boluda JC, Esquirol A, et al. A time-to-event model for acute kidney injury after reduced-intensity conditioning stem cell transplantation using a tacrolimus- and sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transpl. 2017;23:1177–85.
Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, et al. Calcineurin inhibitor-free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transpl. 2014;20:1828–34.
Bradstock KF, Bilmon I, Kwan J, Micklethwaite K, Blyth E, Deren S, et al. Single-agent high-dose cyclophosphamide for graft-versus-host disease prophylaxis in human leukocyte antigen-matched reduced-intensity peripheral blood stem cell transplantation results in an unacceptably high rate of severe acute graft-versus-host disease. Biol Blood Marrow Transpl. 2015;21:941.
Alousi AM, Brammer JE, Saliba RM, Andersson B, Popat U, Hosing C, et al. Phase II trial of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide after reduced-intensity busulfan/fludarabine conditioning for hematological malignancies. Biol Blood Marrow Transpl. 2015;21:906–12.
Gaballa S, Ge I, El Fakih R, Brammer JE, Kongtim P, Tomuleasa C, et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. 2016;122:3316–26.
Mehta RS, Saliba RM, Chen J, Rondon G, Hammerstrom AE, Alousi A, et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol. 2016;173:444–55.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transpl. 2016;22:1037–42.
Jorge AS, Suárez-Lledó M, Pereira A, Gutierrez G, Fernández-Avilés F, Rosiñol L, et al. Single antigen-mismatched unrelated hematopoietic stem cell transplantation using high-dose post-transplantation cyclophosphamide is a suitable alternative for patients lacking HLA-matched donors. Biol Blood Marrow Transpl. 2018;24:1196–202.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
García-Cadenas, I., Awol, R., Esquirol, A. et al. Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transplant 55, 1041–1049 (2020). https://doi.org/10.1038/s41409-019-0771-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0771-2
This article is cited by
-
Outcome improvement over time in reduced intensity conditioning hematopoietic transplantation: a 20-year experience
Annals of Hematology (2024)
-
Real-world data suggest effectiveness of the allogeneic mesenchymal stromal cells preparation MSC-FFM in ruxolitinib-refractory acute graft-versus-host disease
Journal of Translational Medicine (2023)
-
Daratumumab may be the most effective treatment for post-engraftment pure red cell aplasia due to persistent anti-donor isohemagglutinins after major ABO-mismatched allogeneic transplantation
Bone Marrow Transplantation (2022)
-
Systematic overview of HLA-matched allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide
International Journal of Hematology (2022)
-
Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: impact of HLA donor matching
Bone Marrow Transplantation (2021)