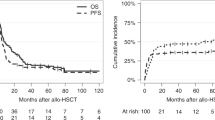
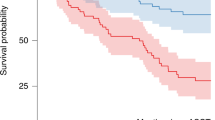
Abstract
Melphalan is given at a dose of 200 mg/m2 (Mel200) prior to ASCT for multiple myeloma (MM). Pharmacokinetic (PK) studies show a high degree of interpatient variability. We aimed to test the impact of clinical factors previously shown to affect melphalan PK such as hemoglobin (Hgb), fat-free mass (FFM), and creatinine clearance (CrCl) on outcomes. Median Hgb (from day −2 to −1) and FFM were grouped as low or high relative to their sample medians, and CrCl was divided into ≥60 or <60 ml/min. In 133 MM patients, median TFS (defined as time from ASCT to initiation of next subsequent line of therapy or death) was longer in patients with lower Hgb (35 vs. 16 months, p = 0.02). Patients with both lower Hgb and CrCl experienced longer TFS compared to those with higher Hgb and CrCl (35 vs. 13 months, p = 0.03). In multivariate analysis, lower hemoglobin, lower CrCl, and a combined low hemoglobin and CrCl were strongly associated with improved TFS. Patients with a lower hemoglobin or creatinine clearance experienced significantly more toxicity. We show for the first time that Hgb and CrCl are important predictors of outcomes after Mel200. PK-directed melphalan dosing may be beneficial in achieving optimal outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Esma F, Salvini M, Troia R, Boccadoro M, Larocca A, Pautasso C. Melphalan hydrochloride for the treatment of multiple myeloma. Expert Opin Pharm. 2017;18:1127–36.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7.
Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–84.
Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, et al. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2015;21:1155–66.
Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99.
Shaw PJ, Nath CE, Lazarus HM. Not too little, not too much-just right! (Better ways to give high dose melphalan). Bone Marrow Transpl. 2014;49:1457–65.
Kuhne A, Tzvetkov MV, Hagos Y, Lage H, Burckhardt G, Brockmoller J. Influx and efflux transport as determinants of melphalan cytotoxicity: resistance to melphalan in MDR1 overexpressing tumor cell lines. Biochem Pharm. 2009;78:45–53.
Nath CE, Shaw PJ, Trotman J, Zeng L. Pharmacokinetics of melphalan in myeloma patients undergoing an autograft. Bone Marrow Transpl. 2007;40:707–8.
Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharm. 2016;82:149–59.
Bubalo J, Carpenter PA, Majhail N, Perales MA, Marks DI, Shaughnessy P, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation Practice Guideline Committee. Biol Blood Marrow Transpl. 2014;20:600–16.
Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharm. 2010;69:484–97.
Cho YK, Sborov DW, Lamprecht M, Li J, Wang J, Hade EM, et al. Associations of high-dose melphalan pharmacokinetics and outcomes in the setting of a randomized cryotherapy trial. Clin Pharm Ther. 2017;102:511–9.
Kuhne A, Sezer O, Heider U, Meineke I, Muhlke S, Niere W, et al. Population pharmacokinetics of melphalan and glutathione S-transferase polymorphisms in relation to side effects. Clin Pharm Ther. 2008;83:749–57.
Mizuno K, Dong M, Fukuda T, Chandra S, Mehta PA, McConnell S, et al. Population pharmacokinetics and optimal sampling strategy for model-based precision dosing of melphalan in patients undergoing hematopoietic stem cell transplantation. Clin Pharm. 2018;57:625–36.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharm. 2005;44:1051–65.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
GlaxoSmithKline. ALKERAN (melphalan) [package insert]. GlaxoSmithKline; NC: Research Triangle Park; 2010.
Nieto Y, Vaughan WP. Pharmacokinetics of high-dose chemotherapy. Bone Marrow Transpl. 2004;33:259–69.
Kuhne A, Kaiser R, Schirmer M, Heider U, Muhlke S, Niere W, et al. Genetic polymorphisms in the amino acid transporters LAT1 and LAT2 in relation to the pharmacokinetics and side effects of melphalan. Pharm Genom. 2007;17:505–17.
Giglia JL, White MJ, Hart AJ, Toro JJ, Freytes CO, Holt CC, et al. A single nucleotide polymorphism in SLC7A5 is associated with gastrointestinal toxicity after high-dose melphalan and autologous stem cell transplantation for multiple myeloma. Biol Blood Marrow Transpl. 2014;20:1014–20.
Reece PA, Hill HS, Green RM, Morris RG, Dale BM, Kotasek D, et al. Renal clearance and protein binding of melphalan in patients with cancer. Cancer Chemother Pharm. 1988;22:348–52.
Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, et al. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996;2:947–52.
Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–9.
Carlson K. Melphalan 200 mg/m2 with blood stem cell support as first-line myeloma therapy: impact of glomerular filtration rate on engraftment, transplantation-related toxicity and survival. Bone Marrow Transpl. 2005;35:985–90.
Parikh GC, Amjad AI, Saliba RM, Kazmi SM, Khan ZU, Lahoti A, et al. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transpl. 2009;15:812–6.
Sweiss K, Patel S, Culos K, Oh A, Rondelli D, Patel P. Melphalan 200 mg/m(2) in patients with renal impairment is associated with increased short-term toxicity but improved response and longer treatment-free survival. Bone Marrow Transpl. 2016;51:1337–41.
Aljitawi OS, Ganguly S, Abhyankar SH, Ferree M, Marks R, Pipkin JD, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transpl. 2014;49:1042–5.
Dhakal B, D’Souza A, Lakshman A, Hamadani M, Chhabra S, Thompson R, et al. Pharmacokinetics of high-dose propylene glycol-free melphalan in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2018;24:1610–4.
Acknowledgements
GSC was supported by the National Institutes of Health, National Heart, Lung and Blood Institute through grant number R21HL140531.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PRP receives honoraria from Celgene, Janssen, and Amgen and consulting fees from Celgene and Amgen.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sweiss, K., Calip, G.S., Johnson, J.J. et al. Pretransplant hemoglobin and creatinine clearance correlate with treatment-free survival after autologous stem cell transplantation for multiple myeloma. Bone Marrow Transplant 54, 2081–2087 (2019). https://doi.org/10.1038/s41409-019-0628-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0628-8