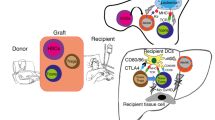
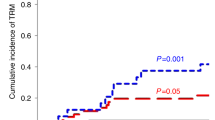
Abstract
Allogeneic hematopoietic cell transplantation from a human leukocyte antigen (HLA) haplotype mismatched donor (haploidentical transplantation) was not feasible for the treatment of hematologic malignancies until the early 1990s, due to the high risk of rejection and graft-versus-host disease (GVHD). The first successful protocol of haploidentical transplantation was based on a highly myeloablative and immunosuppressive conditioning regimen and the infusion of a “mega-dose” of T-cell-depleted hematopoietic stem cells. More than 90% of patients engrafted and <10% developed GVHD. The protocol did not include post-transplant immunosuppression, which favored the graft-versus-tumor effect mediated by alloreactive NK cells and residual alloreactive T cells. However, donor post-transplant immune reconstitution was slow with a high risk of infection-related mortality. More recently, T-cell-depleted haploidentical transplantation has become the platform for innovative cell therapies that aim to enhance T-cell immunity while preventing adverse reactions against host tissues. One strategy is adoptive immunotherapy with conventional T cells and regulatory T cells. Preclinical studies and clinical trials have proven that regulatory T cells control GVHD caused by co-infused conventional T cells while the graft-versus-tumor effect is retained. The use of regulatory T cells in the absence of any other form of immune suppression allowed for a conventional T cell-mediated full eradication of disease in the vast majority of high-risk acute leukemia patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas’ hematopoietic cell transplantation Oxford, United Kingdom: Wiley-Blackwell; 2009.
Anasetti C, Aversa F, Velardi A. Hematopoietic cell transplantation from human leukocyte antigen partially matched related donors. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, eds. Thomas’ hematopoietic cell transplantation. Oxford, United Kingdom: Wiley-Blackwell; 2009. p. 657–74.
Reisner Y, Hagin D, Martelli MF. Haploidentical hematopoietic transplantation: current status and future perspectives. Blood. 2011;118:6006–17.
Velardi A. Haplo-BMT: which approach? [commentary]. Blood. 2013;121:719–20.
Mancusi A, Ruggeri L, Velardi A. Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood. 2016;128:2616–23.
Bachar–Lustig E, Rachamin N, Li HW, et al. Megadose of T-cell depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat Med. 1995;1:1268–73.
Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical” three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–55.
Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93.
Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54.
Martelli MF, Di Ianni M, Ruggeri L, et al. “Designed” grafts for HLA-haploidentical stem cell transplantation. Blood. 2014;123:967–73.
Ciceri F, Labopin M, Aversa F, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112:3574–81.
Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49.
Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9.
Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100.
Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–40.
Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5.
Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–50.
Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–29.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7.
Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9.
Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–9.
Curti A, Ruggeri L, Parisi S, et al. Larger size of donor alloreactive NK cell repertoire correlates with better response to NK cell immunotherapy in elderly acute myeloid leukemia patients. Clin Cancer Res. 2016;22:1914–21.
Stern M, Ruggeri L, Mancusi A, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–5.
Velardi A, Ziagkos D, van Biezen A, et al. Mother donors improve outcomes after HLA haploidentical T cell-depleted hematopoietic transplantation: a Retrospective Study by the Cell Therapy and Immunobiology Working Party of the EBMT. Bone Marrow Transplant. 2016;51(S1):S150. Abstract P076
Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47.
van Kampen CA. Versteeg-van der Voort Maarschalk MF, Langerak-Langerak J, van Beelen E, Roelen DL, Claas FH. Pregnancy can induce long-persisting primed CTLs specific for inherited paternal HLA antigens. Hum Immunol. 2001;62:201–7.
Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4.
Edinger M, Hoffmann P, Ermann J, et al. CD4 + CD25 + regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50.
Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–56.
Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53.
Pierini A, Colonna L, Alvarez M, et al. Donor requirements for regulatory T cell suppression of murine graft-versus-host disease. J Immunol. 2015;195:347–55.
Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–9.
Pierini A, Nishikii H, Baker J, et al. Foxp3+ regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat Commun. 2017;8:15068.
Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8.
Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–44.
Mancusi A, Ruggeri L, Velardi A. Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood. 2016;128:2616–23.
Del Papa B, Ruggeri L, Urbani E, et al. linical-grade-expanded regulatory T cells prevent graft-versus-host disease while allowing a powerful T cell-dependent graft-versus-leukemia effect in murine models. Biol Blood Marrow Transplant. 2017;23:1847–51.
Hippen KL1, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41.
Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70.
Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044–51.
Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66.
Trotta E, Bessette PH, Silveria SL, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med. 2018;24:1005–14.
Pierini A, Strober W, Moffett C, et al. TNF-α priming enhances CD4 + FoxP3 + regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128:866–71.
Mancusi A, Piccinelli S, Velardi A, Pierini A. The effect of TNF-α on regulatory T cell function in graft-versus-host disease. Front Immunol. 2018;9:356.
MacDonald KG, Hoeppli RE, Huang Q, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–24.
Pierini A, Iliopoulou BP, Peiris H, et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight. 2017;2:92865.
Funding
Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AP and AV received start-up grants from the Italian Association for Cancer Research (AIRC). The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pierini, A., Ruggeri, L., Mancusi, A. et al. T cell depletion and no post transplant immune suppression allow separation of graft versus leukemia from graft versus host disease . Bone Marrow Transplant 54 (Suppl 2), 775–779 (2019). https://doi.org/10.1038/s41409-019-0597-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0597-y