Abstract
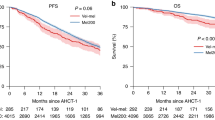
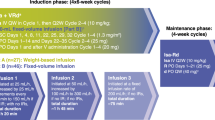
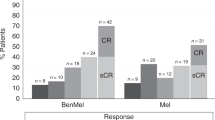
High-dose melphalan (MEL200) followed by autologous stem cell transplantation (ASCT) remains a standard of care for multiple myeloma (MM). Bendamustine induces responses in MM resistant to other alkylators. Our prior Phase I trial adding bendamustine to MEL200 transplant conditioning resulted in no additional toxicity. We now report a single-arm, phase II study that evaluated the efficacy of bendamustine 225 mg/m2 with MEL200 conditioning for ASCT in 18 patients with newly diagnosed MM (NDMM) and 17 with relapsed or refractory MM (RRMM). The primary end point was the complete response (CR/sCR) rate at day+ 100. Sample size was determined according to Simon’s two-stage design. At stage 1, sixteen patients entered the study. As there were eight patients with CR/sCR, enrollment increased to 28 patients. Sixteen out of the first 28 evaluable patients achieved CR/sCR, meeting the design criteria. Enrollment was then expanded to a total of 35 patients. 51% achieved a CR/sCR. After a median follow-up of 65 months, 21 patients progressed, including 7 deaths. The median PFS for NDMM and RRMM was 48 and 45 months, respectively. Bendamustine/MEL200 conditioning resulted in excellent overall and depth of response as well as PFS, particularly in the RRMM patients, and is worthy of further investigation (NCT00916058).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline Summary. J Oncol Pract. 2019;37:1228–63.
Gay F, Engelhardt M, Terpos E, Wasch R, Giaccone L, Auner HW, et al. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica. 2018;103:197–211.
Dhakal B, Szabo A, Chhabra S, Hamadani M, D’Souza A, Usmani SZ, et al. Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: a systematic review and meta-analysis. JAMA Oncol. 2018;4:343–50.
Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv52–iv61.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Moreau P. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
Maybury B, Cook G, Pratt G, Yong K, Ramasamy K. Augmenting autologous stem cell transplantation to improve outcomes in myeloma. Biol Blood Marrow Transplant. 2016;22:1926–37.
Lahuerta JJ, Mateos MV, Martinez-Lopez J, Grande C, de la Rubia J, Rosinol L, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010;95:1913–20.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010;115:32–7.
Roussel M, Hebraud B, Lauwers-Cances V, Macro M, Leleu X, Hulin C, et al. Bortezomib and high-dose melphalan vs. high-dose melphalan as conditioning regimen before autologous stem cell transplantation in de novo multiple myeloma patients: a phase 3 study of the Intergroupe Francophone Du Myelome (IFM 2014-02). Blood. 2017;130(Suppl 1):398.
Blanes M, Lahuerta JJ, González JD, Ribas P, Solano C, Alegre A, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013;19:69–74.
Knop S, Bauer K, Hebart H, Wandt H, Trumper L, Liebisch P, et al. A randomized comparison of total-marrow irradiation, Busulfan and cyclophosphamide with tandem high-dose melphalan in patients with multiple myeloma. Blood. 2007;110:223a–4.
Park SS, Kim K, Kim SJ, Lee JH, Yoon SS, Mun YC, et al. A phase I/II, open-label, prospective, multicenter study to evaluate the efficacy and safety of lower doses of bortezomib plus busulfan and melphalan as a conditioning regimen in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation: The KMM103 study. Biology of blood and marrow transplantation: Biol Blood and Marrow Transplant. 2019.
Beaven AW, Moore DT, Sharf A, Serody JS, Shea TC, Gabriel DA. Infusional mitoxantrone plus bolus melphalan as a stem cell transplant conditioning regimen for multiple myeloma. Cancer Invest. 2011;29:214–9.
Fenk R, Schneider P, Kropff M, Huenerlituerkoglu AN, Steidl U, Aul C, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–94.
Donato ML, Aleman A, Champlin RE, Weber D, Alexanian R, Ippoliti CM, et al. High-dose topotecan, melphalan and cyclophosphamide (TMC) with stem cell support: a new regimen for the treatment of multiple myeloma. Leuk Lymphoma. 2004;45:755–9.
Kazmi SM, Saliba RM, Donato M, Wang M, Hosing C, Qureshi S, et al. Phase II trial of high-dose topotecan, melphalan and CY with autologous stem cell support for multiple myeloma. Bone Marrow Transplant. 2011;46:510–5.
Comenzo RL, Hassoun H, Kewalramani T, Klimek V, Dhodapkar M, Reich L, et al. Results of a phase I/II trial adding carmustine (300 mg/m2) to melphalan (200 mg/m2) in multiple myeloma patients undergoing autologous stem cell transplantation. Leukemia. 2006;20:345–9.
Nieto Y, Valdez BC, Pingali SR, Bassett R, Delgado R, Nguyen J, et al. High-dose gemcitabine, busulfan, and melphalan for autologous stem-cell transplant in patients with relapsed or refractory myeloma: a phase 2 trial and matched-pair comparison with melphalan. Lancet Haematol. 2017;4:e283–92.
Mark TM, Guarneri D, Forsberg P, Rossi A, Pearse R, Perry A, et al. A Phase I trial of high-dose lenalidomide and melphalan as conditioning for autologous stem cell transplantation in relapsed or refractory multiple myeloma. Biol Blood Marrow Transplant. 2017;23:930–7.
Patel P, Oh AL, Koshy M, Sweiss K, Saraf SL, Quigley JG, et al. A phase 1 trial of autologous stem cell transplantation conditioned with melphalan 200 mg/m(2) and total marrow irradiation (TMI) in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2018;59:1666–71.
Costa LJ, Landau HJ, Chhabra S, Hari P, Innis-Shelton R, Godby KN, et al. Phase 1/2 trial of carfilzomib plus high-dose melphalan preparative regimen for salvage autologous hematopoietic cell transplantation followed by maintenance carfilzomib in patients with relapsed/refractory multiple myeloma. Biol Blood Marrow Transplant. 2018;24:1379–85.
Mark TM, Reid W, Niesvizky R, Gergis U, Pearse R, Mayer S, et al. A phase 1 study of bendamustine and melphalan conditioning for autologous stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2013;19:831–7.
Bashir Q, Thall PF, Milton DR, Fox PS, Kawedia JD, Kebriaei P, et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Haematol. 2019;6:E266–75.
Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–64.
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35.
Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–7.
Gandhi V, Burger JA. Bendamustine in B-cell malignancies: the new 46-year-old kid on the block. Clin Cancer Res. 2009;15:7456–61.
Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs. 1996;7:415–21.
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17.
Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(Suppl 1):S12–23.
Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica. 2005;90:1287–8.
Ponisch W, Holzvogt B, Plotze M, Andrea M, Bourgeois M, Heyn S, et al. Bendamustine and prednisone in combination with bortezomib (BPV) in the treatment of patients with newly diagnosed/untreated multiple myeloma. J Cancer Res Clin Oncol. 2014;140:1947–56.
Fenk R, Michael M, Zohren F, Graef T, Czibere A, Bruns I, et al. Escalation therapy with bortezomib, dexamethasone and bendamustine for patients with relapsed or refractory multiple myeloma. Leuk Lymphoma. 2007;48:2345–51.
Ludwig H, Kasparu H, Leitgeb C, Rauch E, Linkesch W, Zojer N, et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014;123:985–91.
Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–13.
Sivaraj D, Green MM, Kang Y, Long GD, Rizzieri DA, Li Z, et al. Bendamustine, pomalidomide, and dexamethasone for relapsed and/or refractory multiple myeloma. Blood Cancer J. 2018;8:71.
Gramatzki M, Günther A, Offidani M, Engelhardt M, Corradini P, Gentili S, et al. Carfilzomib in combination with bendamustine and dexamethasone (CBd) in relapsed and/or refractory patients with multiple myeloma: the phase I/II EMN09 Study. Blood. 2016;128:3334.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031–5.
CIBMTR. Instructions for Post-Transplant Essential Data (Post-TED) Form 2012. https://www.cibmtr.org/DataManagement/TrainingReference/Manuals/DataManagement/Documents/post-ted-instruction.pd.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin trials. 1989;10:1–10.
Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–46.
Storch E, Mark T, Avecilla S, Pagan C, Rhodes J, Shore T, et al. A novel hematopoietic progenitor cell mobilization and collection algorithm based on preemptive CD34 enumeration. Transfusion. 2015;55:2010–6.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–96.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–92.
Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr., Hofmeister CC, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013;54:1658–64.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Bahlis N, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M, et al. Three-year follow up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (d-rd) versus lenalidomide and dexamethasone (rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood. 2018;132(Suppl 1):1996.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66.
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103:2079–97.
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–81.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:728–34.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Dimopoulos MA, Lonial S, Betts KA, Chen C, Zichlin ML, Brun A, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer. 2018;124:4032–43.
Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811–22.
Cavo M, Hájek R, Pantani L, Beksac M, Oliva S, Dozza L, et al. Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood. 2017;130(Suppl 1):397.
Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone--a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J cancer Res Clin Oncol. 2006;132:205–12.
Funding
PC was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384–01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gomez-Arteaga, A., Mark, T.M., Guarneri, D. et al. High-dose bendamustine and melphalan conditioning for autologous stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant 54, 2027–2038 (2019). https://doi.org/10.1038/s41409-019-0587-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0587-0
This article is cited by
-
Adding bendamustine to melphalan before ASCT improves CR rate in myeloma vs. melphalan alone: A randomized phase-2 trial
Bone Marrow Transplantation (2022)
-
Initial Therapeutic Approaches to Patients with Multiple Myeloma
Advances in Therapy (2021)