Abstract
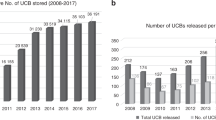
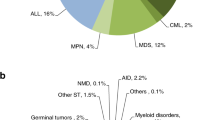
The first hematopoietic transplant in which umbilical cord blood (UCB) was used as the source of hematopoietic cells was performed in October 1988. Since then, significant achievements have been reported in terms of our understanding of the biology of UCB-derived hematopoietic stem (HSCs) and progenitor (HPCs) cells. Over 40,000 UCB transplants (UCBTs) have been performed, in both children and adults, for the treatment of many different diseases, including hematologic, metabolic, immunologic, neoplastic, and neurologic disorders. In addition, cord blood banking has been developed to the point that around 800,000 units are being stored in public banks and more than 4 million units in private banks worldwide. During these 30 years, research in the UCB field has transformed the hematopoietic transplantation arena. Today, scientific and clinical teams are still working on different ways to improve and expand the use of UCB cells. A major effort has been focused on enhancing engraftment to potentially reduce risk of infection and cost. To that end, we have to understand in detail the molecular mechanisms controlling stem cell self-renewal that may lead to the development of ex vivo systems for HSCs expansion, characterize the mechanisms regulating the homing of HSCs and HPCs, and determine the relative place of UCBTs, as compared to other sources. These challenges will be met by encouraging innovative research on the basic biology of HSCs and HPCs, developing novel clinical trials, and improving UCB banking both in the public and private arenas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gluckman E, Broxmeyer HE, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution of a patient with Fanconi anemia by means of umbilical cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8.
Smith AR, Wagner JE. Current clinical management of Fanconi anemia. Expert Rev Hematol. 2012;5:513–22.
Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–32.
Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RS, Graves V, Yoder M, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci USA. 1992;89:4109–13.
Wang JCY, Doedens JCY, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–24.
Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–65.
Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, et al. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood. 1994;83:2489–97.
Bock TA, Orlic D, Dunbar CE, Broxmeyer HE, Bodine DM. Improved engraftment of human hematopoietic cells in severe combined immunodeficient (SCID) mice carrying human cytokine transgenes. J Exp Med. 1995;182:2037–43.
Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–91.
Broxmeyer HE. Proliferative, self-renewal, and survival characteristics of cord blood hematopoietic stem and progenitor cells. In: Cord Blood: Biology, Immunology, Banking and Clinical Transplantation. AABB Press; 2004. p. 1–21.
Mayani H. Biological differences between neonatal and adult human hematopoietic stem/progenitor cells. Stem Cells Dev. 2010;19:285–98.
Broxmeyer HE. Inhibiting HDAC for human hematopoietic stem cell expansión. J Clin Invest. 2014;124:2365–8.
Flores-Guzmán P, Fernández-Sánchez V, Mayani H. Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine. Stem Cells Transl Med. 2013;2:830–8.
Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43:498–513.
Mehta RS, Rezvani K, Olson A, Oran B, Hosing C, Shah N, et al. Novel techniques for ex vivo expansion of cord blood: clinical trials. Front Med. 2015; 2: 89. https://doi.org/10.3389/fmed.2015.00089
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42.
Rebelatto CK, Aguiar AM, Moretao MP. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901–13.
Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, Flores-Guzman P, Hernandez-Estevez E, Fajardo-Orduña G, et al. Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns, and neural protein expression. Cytotherapy. 2009;11:163–76.
Kogler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35.
Wagner JE, Broxmeyer HE, Byrd RL, Zehnbauer B, Schmeckpeper B, Shah N, et al. Transplantation of umbilical cord blood after myeloablative therapy: analysis of engraftment. Blood. 1992;79:1874–81.
Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical cord blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346:214–9.
Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–7.
Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry. Evol Treat Landsc Stem Cells Transl Med. 2018;7:643–50.
National Marrow Donor Program. Unrelated donor search process, step by step. Minneapolis, MN: National Marrow Donor Program; 2009.
Wernet PW. The international NETCORD foundation. In: Broxmeyer HE, (ed.) Cord Blood: Biology, Immunology, Banking and clinical transplantation. Bethesda, MD: AABB Press; 2004. p. 429–35.
Navarrete C, Contreras M. Cord blood banking: a historical perspective. Brit J Haematol. 2009;147:236–45.
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22.
Ballen K, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8.
Rocha V, Kabbara N, Ionescu I, Ruggeri A, Purtill D, Gluckman E. Pediatric related and unrelated cord blood transplantation for malignant diseases. Bone Marrow Transpl. 2009;44:653–9.
Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukemia: a comparison study. Lancet. 2007;369:1947–54.
Prasad VK, Kurtzberg J. Umbilical cord blood transplantation for nonmalignant diseases. Bone Marrow Transpl. 2009;44:643–51.
Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol. 2009;147:246–61.
Ooi J. Cord blood transplantation in adults. Bone Marrow Transpl. 2009;44:661–6.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncol. 2010;11:653–60.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical cord blood or bone marrow from unrelated donors in adults with acute leukemia. New Engl J Med. 2004;351:2276–85.
Munoz J, Shah N, Rezvani K, Hosing C, Bollard CM, Oran B, et al. Concise review: umbilical cord blood transplantation: past, present and future. Stem Cells Transl Med. 2014;3:1435–43.
Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation—from niche to bedside. Nat Rev Clin Oncol. 2015;12:163–74.
Mehta RS, Dave H, Bollard CM, Shpall EJ. Engineering cord blood to improve engraftment after cord blood transplant. Stem Cell Invest. 2017;4:41.
Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1.
Sideri A, Neokleous N, Brunet de la Grange P, Guerton B, Le Bousse Kerdilles MC, Uzan G, et al. An overview of the progress on umbilical cord blood transplantation. Haematologica. 2011;96:1213–20.
Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–8.
Labopin M, Ruggeri A, Gorin NC, Gluckman E, Blaise D, Mannone L, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99:535–40.
Ramirez P, Wagner JE, DeFor TE, Blazar BR, Verneris MR, Miller JS, et al. Factors predicting single-unit predominance after double cord blood transplantation. Bone Marrow Transpl. 2012;47:799–803.
Magro E, Regidor C, Cabrera R, Sanjuan I, Fores R, Garcia-Marco JA, et al. Early hematopoietic recovery after single unit unrelated cord blood transplantation in adults supported by co-infusion of mobilized stem cells from a third party donor. Haematologica. 2006;91:640–8.
Bautista G, Cabrera JR, Regidor C, Fores R, Garcia-Marco JA, Ojeda E, et al. Cord blood transplants supported by co-infusion of mobilized by hematopoietic stem cells from a third party donor. Bone Marrow Transpl. 2009;43:365–73.
Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–45.
Sanchez ME, Ponce DM, Lauer E. Double-unit cord blood (CB) transplantation (DCBT) combined with haplo-identical peripheral blood CD34+ cells (HaploCD34) is associated with enhanced neutrophil recovery, universal haplo rejection, and frequent pre-engraftment syndrome. Biol Blood Marrow Transpl. 2015;21:S43–S44.
Kosuri S, Wolff T, Devlin SM, Byam C, Mazis CM, Naputo K, et al. Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: practical implications for donor selection. Biol Blood Marrow Transpl. 2017;23:965–70.
Metcalf D. Hematopoietic cytokines. Blood. 2008;111:481–5.
Mayani H, Dragowska W, Lansdorp PM. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood. 1993;81:3252–8.
Cardoso A, Li M_L, Batard P. Release from quiescence of CD34+ CD38- human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci USA. 1993;90:8707–11.
Cicuttini FM, Welch KL, Boyd AW. The effect of cytokines on CD34+ Rh-123high and low progenitor cells from human umbilical cord blood. Exp Hematol. 1994;22:1244–51.
Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410–7.
Mayani H, Lansdorp PM. Proliferation of individual hematopoietic progenitors purified from umbilical cord blood. Exp Hematol. 1995;23:1453–62.
de Wynter EA, Nadali G, Coutinho L, Testa NG. Extensive amplification of single cells from CD34+ subpopulations in umbilical cord blood and identification of long-term culture-initiating cells present in two subsets. Stem Cells. 1996;14:566–76.
Piacibello W, Sanavio F, Garetto L, Aglieta M. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–53.
Scadden DT. The stem cell niche as an entity of action. Nature. 2006;441:1075–9.
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611.
Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32:315–20.
Rosler E, Brandt J, Chute J, Hoffman R. Cocultivation of umbilical cord blood cells with endothelial cells leads to extensive amplification of competent CD34+ CD38- cells. Exp Hematol. 2000;28:841–52.
Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transpl. 2006;37:359–66.
Fei XM, Wu YJ, Chang Z, Miao KR, Tang YH, Zhou XY, et al. Co-culture of cord blood CD34+ cells with human BM mesenchymal stromal cells enhances short-term engraftment of cord blood cells in NOD/SCID mice. Cytotherapy. 2007;9:338–47.
Flores-Guzman P, Flores-Figueroa E, Montesinos JJ, Martinez-Jaramillo G, Fernandez-Sanchez V, Valencia-Plata I, et al. Individual and combined effects of mesenchymal stromal cells and recombinant stimulatory cytokines on the in vitro growth of primitive hematopoietic cells from human umbilical cord blood. Cytotherapy. 2009;11:886–96.
Peled T, Mandel J, Goudsmid RN, Landor C, Hasson N, Harati D, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:244–55.
Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6.
Fernandez-Sanchez V, Pelayo R, Flores-Guzman P, Flores-Figueroa E, Villanueva-Toledo J, Garrido E, et al. In vitro effects of stromal cells expressing different levels of Jagged-1 and Delta-1 on the growth of primitive and intermediate CD34+ cell subsets from human cord blood. Blood Cells Mol Dis. 2011;47:205–13.
Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342–55.
Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansión of human hematopoietic stem cells. Science. 2010;329:1345–8.
Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509–12.
Huang X, Lee MR, Cooper S, Hangoc G, Hong KS, Chung HM, et al. Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression. Leukemia. 2015;30:144–53.
Guo B, Huang X, Lee MR, Lee SA, Broxmeyer HE. Antagonism of PPAR-γ signaling expands hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med. 2018;24:36–7.
Chaurasia P, Gajzer DC, Schaniel C, D’Souza S, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395.
Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Bone Marrow Transpl. 2002;8:368–76.
Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase I trial using the AastromReplicell System. Blood. 2003;101:5061–7.
De Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tertraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transpl. 2008;41:771–8.
De Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. New Engl J Med. 2012;367:2305–15.
Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121–8.
Wagner JE, Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, et al. Phase I/II trial of Stem Regenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–55.
Dircio-Maldonado R, Flores-Guzman P, Corral-Navarro J, Mondragon-Garcia I, Hidalgo-Miranda A, Beltran-Anaya FO, et al. Functional integrity and gene expression profiles of human cord blood-derived hematopoietic stem and progenitor cells generated in vitro. Stem Cells Transl Med. 2018;7:602–14.
Horwitz ME, Wease S, Blackwell B, Valcarcel D, Frassoni F, Boelens JJ, et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. https://doi.org/10.1200/JCO.18.00053; 2018.
Heazlewood SY, Oteiza A, Cao H, Nilsson SK. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche. Ann NY Acad Sci. 2014;1310:119–28.
Broxmeyer HE. Enhancing the efficacy of engraftment of cord blood for hematopoietic cell transplantation. Transfus Apher Sci. 2016;54:364–72.
Van OsR, Ausema A, Dontje B, van Riesen M, van Dam G, de Hann G. Engraftment of syngeneic bone marrow is not more efficient after intrafemoral transplantation than after traditional intravenous administration. Exp Hematol. 2010;38:1115–23.
Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transpl. 2009;43:935–40.
Frassoni F, Varaldo R, Gualandi F, Bacigalupo A, Sambuceti G, Sacchi N, et al. The intra-bone marrow injection of cord blood cells extends the possibility of transplantation to the majority of patients with malignant hematopoietic diseases. Best Pract Res Clin Haematol. 2010;23:237–44.
Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/DPPIV regulates CXCL12/SDF-1α mediated chemotaxis of human CD34+ progenitor cells. J Immunol. 2002;169:7000–8.
Christopherson KW, Hangoc G, Mantel C, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3.
Farag SS, Srivastava S, Messina-Graham S, Schwartz J, Robertson MJ, Abonour R, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013;22:1007–15.
Velez de Mendizabal N, Strother RM, Farag SS, Broxmeyer HE, Messina-Graham S, Chitnis SA, et al. Modelling the sitagliptin effect on dipeptidyl peptidase-4 activity in adults with haematological malignancies after umbilical cord blood haematopoietic cell transplantation. Clin Pharm. 2014;53:247–59.
Farag SS, Nelson R, Cairo MS, O’Leary HA, Zhang S, Huntley C, et al. High-dose sitagliptin for systemic inhibition of dipeptidylpeptidase-4 to enhance engraftment of single cord umbilical cord blood transplantation. Oncotarget. 2017;8:110350–7.
Xia L, McDaniel JM, Yago T, Doeden A, Mcever RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–6.
Popat U, Mehta RS, Rezvani K, Fox P, Kondo K, Marin D, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood. 2015;125:2885–92.
Pelus LM, Broxmeyer HE, Kurland JI, Moore MA. Regulation of macrophage and granulocyte proliferation: specificities of prostaglandin E and lactoferrin. J Exp Med. 1979;150:277–92.
North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11.
Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–55.
Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–81.
Brunstein CG, McKenna DH, DeFor TE, Sumstad D, Paul P, Wiesdorf DJ, et al. Complement fragment 3a priming of umbilical cord blood progenitors: safety profile. Biol Blood Marrow Transpl. 2013;19:1474–9.
Capitano ML, Hangoc G, Cooper S, Broxmeyer HE. Mild heat treatment primes human CD34+ cord blood cells for migration towards SDF-1α and enhances engraftment in an NSG mouse model. Stem Cells. 2015;33:1975–84.
Huang X, Guo B, Liu S, Wan J, Broxmeyer HE. Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun. 2018;9:2741 https://doi.org/10.1038/s41467-018-05178-5
Guo B, Huang X, Cooper S, Broxmeyer HE. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med. 2017;23:424–8.
Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A, Schmid C, et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:1810–22.
Bart T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. Clin Outcomes Res. 2010;2:141–7.
Majhail NS, et al. Cost of pediatric allogeneic hematopoietic-cell transplantation. Pedia Blood Cancer. 2010;54:138–43.
Broxmeyer HE, Farag S. Background and future considerations for human cord blood hematopoietic cell transplantation, including economic concerns. Stem Cells Dev. 2013;22(suppl 1):103–10.
Bari S, Zhong Q, Fan X, Poon Z, Lim AST, Lim TH, et al. Ex vivo expansion of CD34+ CD90+ CD49f+ hematopoietic stem and progenitor cells from non-enriched umbilical cord blood with azole compounds. Stem Cells Transl Med. 2018;7:376–93.
Mokhtari S, Baptista PM, Vyas DA, Freeman CJ, Moran E, Brovold M, et al. Evaluating interaction of cord blood hematopoietic stem/progenitor cells with functionally integrated three-dimensional microenvironments. Stem Cells Transl Med. 2018;7:271–82.
Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218–29.
Kurtzberg J, Buntz S, Gentry T, Noeldner P, Ozamiz A, Rusche B, et al. Preclinical characterization of DUOC-01, a cell therapy product derived from banked umbilical cord blood for use as an adjuvant to umbilical cord blood transplantation for treatment of inherited metabolic diseases. Cytotherapy. 2015;17:803–15.
Sun JM, Kurtzberg J. Cell therapy for diverse central nervous system disorders: inherited metabolic diseases and autism. Pedia Res. 2018;83:364–71.
Saha A, Buntz S, Scotland P, Xu L, Noeldner P, Patel S, et al. A cord blood monocyte-derived cell therapy product accelerates brain remyelination. JCI Insight. 2016;1:e86667.
Achyut BR, Varma NR, Arbab AS. Application of umbilical cord blood derived stem cells in diseases of the nervous system. J Stem Cell Res Ther. 2014;4:1000202.
Fleiss B, Guillot PV, Titomanlio L, Baud O, Hagberg H, Gressens P. Stem cell therapy for neonatal brain injury. Clin Perinatol. 2014;41:133–48.
Garbuzova-Davis S, Ehrhart J, Sanberg PR. Cord blood as a potential therapeutic for amyotrophic lateral sclerosis. Expert Opin Biol Ther. 2017;17:837–51.
Chez M, Lepage C, Parise C, Dang-Chu A, Hankins A, Carroll M. Safety and observations from a placebo-controlled, crossover study to assess use of autologous umbilical cord blood stem cells to improve symptoms in children with autism. Stem Cells Transl Med. 2018;7:333–41.
Carpenter KLH, Major S, Tallman C, Chen LW, Franz L, Sun J, et al. White matter tract changes associated with clinical improvement in an open-label trial assessing autologous umbilical cord blood for treatment of young children with autism. Stem Cells Transl Med. 2019;8:138–47.
Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M, et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl Med. 2018;7:521–9.
Park EH, Lim H-S, Lee S, Roh K, Seo KW, Kang KS, et al. Intravenous infusion of umbilical cord blood-derived mesenchymal stem cells in rheumatoid arthritis: a phase 1a clinical trial. Stem Cells Transl Med. https://doi.org/10.1002/sctm.18-0031; 2018.
Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, et al. A randomized placebo-controlled trial of human cord blood-derived mesenchymal stem cell infusion for children with cerebral palsy. Cell Transpl. 2018;27:325–34.
Abo-Elkheir W, Hamza F, Elmofty AM, Emam A, Abdl-Moktader M, Elsherefy S, et al. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study. Am J Stem Cells. 2017;6:23–35.
Ahn SY, Chang YS, Kim JH, Sung SL, Park WS. Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for Bronchopulmonary dysplasia. J Pedia. 2017;185:49–54.
Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, Kim TY. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35:248–55.
Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560.
Mukai T, Nagamura-Inoue T, Shimazu T, Mori Y, Takahashi A, Tsunoda H, et al. Neurosphere formation enhances the neurogenic differentiation potential and migratory ability of umbilical cord-mesenchymal stromal cells. Cytotherapy. 2016;18:229–41.
Donders R, Bogie JFJ, Ravanidis S, Gervois P, Vanheusden M, Marée R, et al. Human Wharton’s jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared to bone marrow-derived stem cells. Stem Cells Dev. 2018;27:65–84.
Singh AK, Kashyap MP, Jahan S, Kumar V, Tripathi VK, Siddiqui MA, et al. Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34+ stem cell-derived differentiating neuronal cells. Toxicol Sci. 2012;129:392–410.
Giorgetti A, Marchetto MC, Li M, Yu D, Fazzina R, Mu Y, et al. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA. 2012;109:12556–61.
Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza L, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–7.
Lee MR, Prasain N, Chae H-D, Kim YJ, Mantel C, Yoder MC, et al. Epigenetic regulation of Nanog by miR-302 cluster-MBD2 completes induced pluripotent stem cell reprogramming. Stem Cells. 2013;31:666–81.
Acknowledgements
Research in the Mayani laboratory is supported by grants from the Mexican Institute of Social Security (IMSS) and the National Council of Science and Technology (CONACYT), Mexico. Publications reported from the Broxmeyer lab were supported by Public Health Service Grants from the National Institutes of Health: R35 HL139599, R01 DK109188, R01 HL056416, R01 HL112669, and U54 DK106846.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayani, H., Wagner, J.E. & Broxmeyer, H.E. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant 55, 48–61 (2020). https://doi.org/10.1038/s41409-019-0546-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0546-9
This article is cited by
-
Cord Blood Transplantation for Very Early-Onset Inflammatory Bowel Disease Caused by Interleukin-10 Receptor Deficiency
Journal of Clinical Immunology (2024)
-
Projected Impact of Omidubicel-onlv on Racial/Ethnic Disparities in Allogeneic Hematopoietic Cell Transplantation (Allo-HCT) Outcomes in Hematologic Malignancies
Advances in Therapy (2024)
-
A predictive model combining clinical characteristics and nutritional risk factors for overall survival after umbilical cord blood transplantation
Stem Cell Research & Therapy (2023)
-
Outcomes of graft failure after umbilical cord blood transplantation in acute leukemia: a study from Eurocord and the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Hybrid umbilical cord blood banking: literature review
Archives of Gynecology and Obstetrics (2023)