Abstract
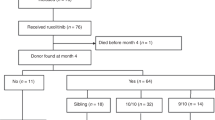
Ruxolitinib (Rux), a Jak1/2 inhibitor, results in reduced spleen size and improvement in constitutional symptoms in the majority of patients with myelofibrosis (MF). Therefore Rux, when given prior to hematopoietic cell transplantation (HCT) in patients with MF was hypothesized to improve engraftment, decrease incidence and severity of graft-versus-host disease, and lower non-relapse mortality (NRM). We conducted a phase II prospective trial to assess the effects of pre-HCT Rux on post-HCT outcomes in patients with MF. The primary endpoint was 2-year overall survival. To date, 28 patients (median age 56 years) have been transplanted. The median time on Rux pre-HCT was 7 months. Twenty-three patients received myeloablative and five reduced intensity conditioning. Donors included 14 HLA-matched siblings, 11 matched unrelated, 1 allele mismatched unrelated, and 3 umbilical cord blood. There have been no episodes of cytokine release syndrome and all patients achieved sustained engraftment. Two patients died from NRM and two patients relapsed. With a median follow-up of 13 months, overall survival is 93% (95% CI: 0.73, 0.98) at 1 year and 86% (95% CI: 0.61, 0.96) at 2 years post-HCT. This study demonstrates that pre-HCT Rux is well tolerated and suggests that pre-HCT Rux may improve post-HCT outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Scott BL, Gooley TA, Sorror ML, Rezvani AR, Linenberger ML, Grim J, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119:2657–64.
Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–33.
Samuelson Bannow BT, Salit RB, Storer BE, Stevens EA, Wu D, Yeung C, et al. Hematopoietic cell transplantation for myelofibrosis: the dynamic International Prognostic Scoring System plus risk predicts post-transplant outcomes. Biol Blood Marrow Transplant. 2018;24:386–92.
Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124:1062–9.
Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–500.
Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–7.
Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–9.
Ditschkowski M, Elmaagacli AH, Trenschel R, Gromke T, Steckel NK, Koldehoff M, et al. Dynamic International Prognostic Scoring System scores, pre-transplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2012;97:1574–81.
Febvre-James M, Lecureur V, Augagneur Y, Mayati A, Fardel O. Repression of interferon beta-regulated cytokines by the JAK1/2 inhibitor ruxolitinib in inflammatory human macrophages. Int Immunopharmacol. 2018;54:354–65.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126:1551–4.
Newberry KJ, Patel K, Masarova L, Luthra R, Manshouri T, Jabbour E, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130:1125–31.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Milano F, Appelbaum FR, Delaney C. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:2204–5.
Rezvani AR, McCune JS, Storer BE, Batchelder A, Kida A, Deeg HJ, et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant. 2013;19:1033–9.
Konuma T, Ooi J, Takahashi S, Tomonari A, Tsukada N, Kato S, et al. Second myeloablative allogeneic stem cell transplantation (SCT) using cord blood for leukemia relapsed after initial allogeneic SCT. Leuk Res. 2009;33:840–2.
Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP, et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood. 2005;105:4115–9.
Takagi S, Ota Y, Uchida N, Takahashi K, Ishiwata K, Tsuji M, et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for myelofibrosis. Blood. 2010;116:649–52.
Jaekel N, Behre G, Behning A, Wickenhauser C, Lange T, Niederwieser D, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis in patients pretreated with the JAK1 and JAK2 inhibitor ruxolitinib. Bone Marrow Transplant. 2014;49:179–84.
Stubig T, Alchalby H, Ditschkowski M, Wolf D, Wulf G, Zabelina T, et al. JAK inhibition with ruxolitinib as pretreatment for allogeneic stem cell transplantation in primary or post-ET/PV myelofibrosis. Leukemia. 2014;28:1736–8.
Lebon DRM, Rubio MT, Legrand F, Kiladjian J, Mohty M, Cahn J et al. Ruxolitinib for patients with primary or secondary myelofibrosis before allogeneic hematopoietic stem cell transplantation (allo-HSCT): a retrospective study of the Societe Franc ßaise De Greffe De Moelle Et De Therapie Cellulaire (SFGM-TC). Blood. 2013;122:2111. (ASH Abstracts).
Shanavas M, Popat U, Michaelis LC, Fauble V, McLornan D, Klisovic R, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to Janus Kinase 1/2 inhibitors. Biol Blood Marrow Transplant. 2016;22:432–40.
Robin MFS, Huynh A, et al. Ruxolitinib before allogeneic hematopoietic stem cell transplantation (HSCT) in patients with myelofibrosis: a preliminary descriptive report of the JAK ALLO Study, a phase II trial sponsored by Goelams-FIM in collaboration with the Sfgmtc. Blood. 2013;122:306 (ASH Abstracts).
Gupta V, Kosiorek HE, Mead A, Klisovic RB, Galvin JP, Berenzon D, et al. Ruxolitinib therapy followed by reduced-intensity conditioning for hematopoietic cell transplantation for myelofibrosis: Myeloproliferative Disorders Research Consortium 114 Study. Biol Blood Marrow Transplant. 2019;25:256–64.
Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff NV. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10:391–402.
Kroger N. Ruxolitinib during peritransplant period for myelofibrosis patients undergoing allogeneic stem cell transplantation reduces acute graft verus host disease. Blood. 2016;128:2242 (ASH Abstracts).
Rajantie J, Sale GE, Deeg HJ, Amos D, Appelbaum F, Storb R, et al. Adverse effect of severe marrow fibrosis on hematologic recovery after chemoradiotherapy and allogeneic bone marrow transplantation. Blood. 1986;67:1693–7.
Soll E, Massumoto C, Clift RA, Buckner CD, Appelbaum FR, Storb R, et al. Relevance of marrow fibrosis in bone marrow transplantation: a retrospective analysis of engraftment. Blood. 1995;86:4667–73.
Robin M, Giannotti F, Deconinck E, Mohty M, Michallet M, Sanz G, et al. Unrelated cord blood transplantation for patients with primary or secondary myelofibrosis. Biol Blood Marrow Transplant. 2014;20:1841–6.
Caocci G, Maccioni A, Murgia F, Perra A, Usai M, Piga M, et al. Modulation of bone marrow microenvironment following ruxolitinib therapy in myelofibrosis. Leuk Lymphoma. 2016;57:1215–8.
Acknowledgements
The authors would like to thank Eileen Sickle and Elizabeth Harrington for their tireless work as the research coordinators and Amita Kaur for her work as a data coordinator on this study. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA015704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salit, R.B., Scott, B.L., Stevens, E.A. et al. Pre-hematopoietic cell transplant Ruxolitinib in patients with primary and secondary myelofibrosis. Bone Marrow Transplant 55, 70–76 (2020). https://doi.org/10.1038/s41409-019-0523-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0523-3
This article is cited by
-
The application of JAK inhibitors in the peri-transplantation period of hematopoietic stem cell transplantation for myelofibrosis
Annals of Hematology (2024)
-
The role of JAK inhibitors in hematopoietic cell transplantation
Bone Marrow Transplantation (2022)
-
Improving allogeneic stem cell transplantation in myelofibrosis
International Journal of Hematology (2022)
-
Current Management of Chronic Neutrophilic Leukemia
Current Treatment Options in Oncology (2021)
-
Impact of prior JAK-inhibitor therapy with ruxolitinib on outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis: a study of the CMWP of EBMT
Leukemia (2021)