Abstract
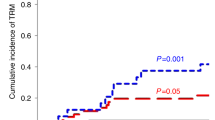
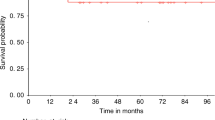
In previous studies, we and others observed in patients undergoing HLA-matched hematopoietic cell transplantation that high proportion of donor-derived CD4+/CCR7+ T cells were associated with an increased risk of acute GVHD without any interference in relapse incidence. We investigated the impact of donor-derived CD4+/CCR7+ T cells on patient outcome in haploidentical settings where posttransplant cyclophosphamide is used. We analyzed T-cell subsets in grafts of 29 adult patients who underwent first haploidentical transplant following reduced intensity conditioning. The median CD4+/CCR7+ subset proportion was 69.2% among donor CD4+ T cells. With a median follow-up of 28.1 months (range: 11.0–44.3), 16 patients (55%) developed acute GVHD; this includes 5 patients with grade 3 acute GVHD. Fifty-four percent of patients who received > 69.2% of CD4+/CCR7+ T cells and 12% of patients who received < 69.2% CD4+/CCR7+ T cells developed acute GVHD (p = 0.028). In multivariate analysis, a high proportion of CD4+/CCR7+ T cells was the only factor that impacted acute GVHD (HR = 4.925, 95% CI [1.020–23.775], p = 0,047) with no impact on overall survival. Our results confirm the impact of a high proportion of CD4+/CCR7+ T cells on acute GVHD incidence in patients undergoing haploidentical transplant despite the use of posttransplant cyclophosphamide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ciurea SO, Bayraktar UD. “No donor”? Consider a haploidentical transplant. Blood Rev. 2015;29:63–70.
Yakoub-Agha I, Saule P, Depil S, Micol J-B, Grutzmacher C, Boulanger-Villard F, et al. A high proportion of donor CD4+T cells expressing the lymph node-homing chemokine receptor CCR7 increases incidence and severity of acute graft-versus-host disease in patients undergoing allogeneic stem cell transplantation for hematological malignancy. Leukemia. 2006;20:1557–65.
Portero-Sainz I, Gómez-García de Soria V, Cuesta-Mateos C, Fernández-Arandojo C, Vega-Piris L, Royg M, et al. A high migratory capacity of donor T-cells in response to the lymph node homing receptor CCR7 increases the incidence and severity of GvHD. Bone Marrow Transplant. 2017;52:745–52.
Varlet P, Rogeau S, Trauet J, Demaret J, Labalette M. Immunomagnetic selective donor-derived CD4+CCR7+T cell depletion procedure for peripheral blood stem cells graft. Curr Res Transl Med. 2019;67:1-7 https://linkinghub.elsevier.com/retrieve/pii/S2452318618300552
Choufi B, Trauet J, Thiant S, Labalette M, Yakoub-Agha I. Donor-derived CD4(+)/CCR7(+) T-cell partial selective depletion does not alter acquired anti-infective immunity. Bone Marrow Transplant. 2014;49:611–5.
Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–8.
Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103:1534–41.
Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, West ML, Burgents JE, Blazar BR, et al. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115:4914–22.
Dubois V, Alizadeh M, Bourhis JH, Etancelin P, Farchi O, Ferrand C, et al. Étude du chimérisme après allogreffe de cellules hématopoïétiques: recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC). Bull Cancer (Paris). 2017;104:S59–64.
Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Rich JT, Neely JG, Paniello RC, Voelker CCJ, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngol-Head Neck Surg. 2010 Sep;143:331–6.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50(S2):S37–9.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant . 2015;21:389–401.e1.
Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell . 1999;99:23–33.
Ferrara JLM, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10.
Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–22.
Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835–44.
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93.
Mussetti A, De Philippis C, Carniti C, Bastos Oreiro M, Gayoso J, Cieri N, et al. CD3 lymphocyte graft content and not hematopoietic stem cell source is associated to higher chronic GVHD after haploidentical transplantation with post-transplant cyclophosphamide. Blood. 2017;130(Suppl 1):4587.
Alwasaidi T, Bredeson C. Peripheral blood stem cells or bone marrow as the graft source for allogeneic hematopoietic cell transplantation? J Taibah Univ Med Sci. 2014;9:91–9.
Xystrakis E, Bernard I, Dejean AS, Alsaati T, Druet P, Saoudi A. Alloreactive CD4 T lymphocytes responsible for acute and chronic graft-versus-host disease are contained within the CD45RChigh but not the CD45RClow subset. Eur J Immunol. 2004;34:408–17.
Retière C, Willem C, Guillaume T, Vié H, Gautreau-Rolland L, Scotet E, et al. Impact on early outcomes and immune reconstitution of high-dose post-transplant cyclophosphamide vs anti-thymocyte globulin after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation. Oncotarget. 2018;9:11451–64.
Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125:2855–64.
Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–61.
Melenhorst JJ, Scheinberg P, Williams A, Ambrozak DR, Keyvanfar K, Smith M, et al. Alloreactivity across HLA barriers is mediated by both naïve and antigen-experienced T cells. Biol Blood Marrow Transplant. 2011;17:800–9.
Al-Homsi AS, Goodyke A, McLane M, Abdel-Mageed S, Cole K, Muilenburg M, et al. Post-transplantation cyclophosphamide and ixazomib combination rescues mice subjected to experimental graft-versus-host disease and is superior to either agent alone. Biol Blood Marrow Transplant. 2017;23:255–61.
Atilla E, Atilla PA, Bozdağ SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45:403–11.
McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–8.
Jauréguiberry S, Thellier M, Ndour PA, Ader F, Roussel C, Sonneville R, et al. Delayed-onset hemolytic anemia in patients with travel-associated severe malaria treated with artesunate, France, 2011-2013. Emerg Infect Dis. 2015;21:804–12.
Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20:1573–9.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–22.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai H-L, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9.
Acknowledgements
We thank all our patients and donors who participated in this study. We thank the Association Capucine for supporting our research. A special thanks to Rachel Tipton for checking the English of the manuscript.
Author information
Authors and Affiliations
Contributions
Designed and performed research: PV, TA, JT, and IYA. Analyzed and interpreted data: PV, TA, and IYA. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varlet, P., Alsuliman, T., Trauet, J. et al. Donor-derived CD4+/CCR7+ T-cell impact on acute GVHD incidence following haplo-HCT after reduced intensity conditioning and posttransplant cyclophosphamide. Bone Marrow Transplant 54, 1686–1693 (2019). https://doi.org/10.1038/s41409-019-0511-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0511-7
This article is cited by
-
Evaluation of therapeutic targeting of CCR7 in acute graft-versus-host disease
Bone Marrow Transplantation (2020)