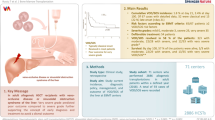
Abstract
Traditional severity criteria of sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) were determined retrospectively but found inappropriate for therapeutic decisions. Data of 203 patients with SOS/VOD were collected according to the modified Seattle diagnostic criteria and were analyzed for validation of the revised severity criteria recently proposed from European Society for Blood and Marrow Transplantation (EBMT). According to the traditional severity criteria, none of the patients were mild grade, while 63.1% were moderate and 36.9% were severe grade. However, according to the revised EBMT criteria, the majority of patients (63.1%) were very severe, 18.2% were severe, 12.8% were moderate, and 5.9% were mild grade. The 100-day overall survival (OS) of mild, moderate, severe and very severe groups was 83.3, 84.3, 94.6, and 58.6%, respectively. Very severe SOS/VOD showed a significantly lower OS than the others (58.6 vs. 89.3%, p < 0.0001). The 100-day transplantation-related mortality was 25.2% in the entire cohort; 8.3% in mild, 8.0% in moderate, 2.7% in severe, and 36.7% in very severe SOS/VOD (p < 0.0001). The very severe grade newly classified by the revised EBMT criteria accounted for the majority of SOS/VOD associated with worse 100-day OS. Therefore, intervention should be applied at the latest for moderate to severe SOS/VOD before deterioration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–68. https://doi.org/10.1016/j.bbmt.2009.08.024
Lee SH, Yoo KH, Sung KW, Koo HH, Kwon YJ, Kwon MM, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45:1287–93. https://doi.org/10.1038/bmt.2009.349.
Yoon JH, Min WS, Kim HJ, Kim JH, Shin SH, Yahng SA. et al. Experiences of t-PA use in moderate-to-severe hepatic veno-occlusive disease after hematopoietic SCT: is it still reasonable to use t-PA?. Bone Marrow Transplant.2013;48:1562–8. https://doi.org/10.1038/bmt.2013.101.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10:451–62.
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–36. https://doi.org/10.1200/JCO.1993.11.9.1729
Chao N. How I treat sinusoidal obstruction syndrome. Blood. 2014;123:4023–6. https://doi.org/10.1182/blood-2014-03-551630
Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168:481–91. https://doi.org/10.1111/bjh.13215
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50:781–9. https://doi.org/10.1038/bmt.2015.52
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65. https://doi.org/10.1182/blood-2015-10-676924
Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Antin JH, et al. Defibrotide for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome: interim results from a treatment IND study. Biol Blood Marrow Transplant. 2017;23:997–1004. https://doi.org/10.1016/j.bbmt.2017.03.008
Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Antin JH, et al. Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day+100 survival following haematopoietic stem cell transplantation. Br J Haematol. 2017;178:112–8. https://doi.org/10.1111/bjh.14727
Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181:816–27. https://doi.org/10.1111/bjh.15267
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12. https://doi.org/10.1038/bmt.2016.130
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Miano M, Faraci M, Dini G, Bordigoni P. Early complications following haematopoietic SCT in children. Bone Marrow Transplant. 2008;41:S39–42. https://doi.org/10.1038/bmt.2008.53.
Forrest DL, Thompson K, Dorcas VG, Couban SH, Pierce R. Low molecular weight heparin for the prevention of hepatic veno-occlusive disease (VOD) after hematopoietic stem cell transplantation: a prospective phase II study. Bone Marrow Transplant. 2003;31:1143–9. https://doi.org/10.1038/sj.bmt.1704087.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53:138–45. https://doi.org/10.1038/bmt.2017.161
Acknowledgements
The authors acknowledge Yun Jung Choi, PhD., Rph. and Jeonghee Kim, PhD. from HANDOK Inc. for the coordination of data collection and statistical analysis supervised by the investigators.
Author information
Authors and Affiliations
Contributions
J-H.Y. designed the study, enrolled patients, collected and analyzed data, and wrote the manuscript; K.H.Y., K.W.S., C.W.J., J.S.K., S.M.H., H.J.K., J-H.L., H.J.I., H.K., J.-S.A., and B.C., analyzed data, enrolled patients; J.W.L. designed and conducted the study, enrolled patients, analyzed data, and wrote the manuscript; and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoon, JH., Yoo, K.H., Sung, K.W. et al. Validation of treatment outcomes according to revised severity criteria from European Society for Blood and Marrow Transplantation (EBMT) for sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD). Bone Marrow Transplant 54, 1361–1368 (2019). https://doi.org/10.1038/s41409-019-0492-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0492-6
This article is cited by
-
Utility of the refined EBMT diagnostic and severity criteria 2023 for sinusoidal obstruction syndrome/veno-occlusive disease
Bone Marrow Transplantation (2024)
-
Analysis of laboratory parameters before the occurrence of hepatic sinusoidal obstruction syndrome in children, adolescents, and young adults after hematopoietic stem cell transplantation
Journal of Cancer Research and Clinical Oncology (2024)
-
Transjugular Intrahepatic Portosystemic Shunt Benefits for Hepatic Sinusoidal Obstruction Syndrome Associated with Consumption of Gynura Segetum: a Propensity Score-Matched Analysis
CardioVascular and Interventional Radiology (2023)
-
Current incidence, severity, and management of veno-occlusive disease/sinusoidal obstruction syndrome in adult allogeneic HSCT recipients: an EBMT Transplant Complications Working Party study
Bone Marrow Transplantation (2023)
-
Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT)
Bone Marrow Transplantation (2023)