Abstract
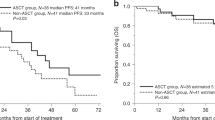
Clinical trials evaluating the role of autologous hematopoietic stem cell transplantation (auto-HCT) in multiple myeloma have mostly included patients aged <65 years. Therefore, this study was aimed to evaluate the efficacy and safety of auto-HCT in elderly patients with multiple myeloma in the era of novel agents. We retrospectively analyzed 2056 patients with multiple myeloma, who underwent auto-HCT in 2007–2014 (287 were aged ≥65 years). We evaluated the 100-day treatment-related mortality (TRM) and overall survival (OS) in two groups; elderly patients ( ≥65 years) who underwent auto-HCT compared with younger patients ( <65 years). In the propensity score–matched-pair analysis used to adjust for possible selection bias, the incidence of 100-day TRM between patients aged <65 (0.4%; 95% confidence interval [CI]: 0.0–2.0%) and ≥65 years (1.2%; 95% CI: 0.3–3.1%) showed no statistically significant difference (p = 0.31). The probability of the 5-year OS after transplantation in those aged <65 (62.5%; 95% CI: 58.6–66.1%) and ≥65 (63.5%; 95% CI: 52.2–72.7%) years was also not significantly different (p = 0.56). This study showed that the safety and efficacy of auto-HCT in elderly patients with multiple myeloma in the era of novel agents compared with younger patients were similar.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Palumbo A, Cavallo F, Gay F, Raimondo DF, Yehuda BD, Petrucci MT. et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905.
Cavo M, Palumbo A, Zweegman S, Dimopoulos MA, Hajek R, Pantani L, et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). J Clin Oncol. 2016;34(15-suppl):8000–8000.
Ozaki S, Harada T, Saitoh T, Shimazaki C, Itagaki M, Asaoku H, et al. Survival of multiple myeloma patients aged 65-70 years in the era of novel agents and autologous stem cell transplantation. A multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014;132:211–9.
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–7.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–18.
Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood. 2010;116:2215–23. e-pub ahead of print 2010/07/21
Klepin HD, Hurd DD. Autologous transplantation in elderly patients with multiple myeloma: are we asking the right questions. Bone Marrow Transplant. 2006;38:585–92. e-pub ahead of print 2006/09/06
Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Blade J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118:4519–29.
Auner HW, Szydlo R, Hoek J, Goldschmidt H, Stoppa AM, Morgan GJ, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transplant. 2015;50:209–15.
Merz M, Neben K, Raab MS, Sauer S, Egerer G, Hundemer M, et al. Autologous stem cell transplantation for elderly patients with newly diagnosed multiple myeloma in the era of novel agents. Ann Oncol. 2014;25:189–95.
Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: results from a matched pair analysis. Am J Hematol. 2008;83:614–7.
Sharma M, Zhang MJ, Zhong X, Abidi MH, Akpek G, Bacher U, et al. Older patients with myeloma derive similar benefit from autologous transplantation. Biol Blood Marrow Transplant. 2014;20:1796–803.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74. e-pub ahead of print 2007/11/09
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10. e-pub ahead of print 2015/11/09
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. e-pub ahead of print 2006/07/21
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23. e-pub ahead of print 1998/09/30
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. e-pub ahead of print 2012/12/05
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74. e-pub ahead of print 2015/01/30
McCarthy PL Jr, Hahn T, Hassebroek A, Bredeson C, Gajewski J, Hale, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19:1116–23.
Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131:301–10. e-pub ahead of print 2017/11/19
Takezako N, Tokuhira M, Sekiguchi N, Kurihara Y, Ito K, Kurimoto M, et al. The efficacy and safety of weekly bortezomib containing VMP followed by bortezomib maintenance therapy in unfit or frail multiple myeloma patients. Clin Lymph Myeloma Leuk. 2016;128:4529.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31. e-pub ahead of print 2016/10/06
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66. e-pub ahead of print 2016/08/25
Merz M, Jansen L, Castro FA, Hillengass J, Salwender H, Weisel K, et al. Survival of elderly patients with multiple myeloma-effect of upfront autologous stem cell transplantation. Eur J Cancer. 2016;62:1–8. e-pub ahead of print 2016/05/18
Stettler J, Novak U, Baerlocher GM, Seipel K, Mansouri Taleghani B, Pabst T. Autologous stem cell transplantation in elderly patients with multiple myeloma: evaluation of its safety and efficacy. Leuk Lymphoma. 2017;58:1076–83. e-pub ahead of print 2016/10/14
Huang LW, Bacon W, Cirrincione C, Peterson B, Long G, Rizzieri D, et al. Efficacy and safety of high-dose chemotherapy with autologous stem cell transplantation in senior versus younger adults with newly diagnosed multiple myeloma. Hematol Oncol. 2017;35:752–9. e-pub ahead of print 2017/01/21
Costa LJ, Zhang MJ, Zhong X, Dispenzieri A, Lonial S, Krishnan A, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615–24. e-pub ahead of print 2013/08/14
Palumbo A, Sezer O, Kyle R, Miguel JS, Orlowski RZ, Moreau P, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009;23:1716–30. e-pub ahead of print 2009/06/06
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet (Lond, Engl). 2007;370:1209–18. e-pub ahead of print 2007/10/09
Ghilardi G, Pabst T, Jeker B, Muller R, Cairoli A, Muller AMS, et al. Melphalan dose in myeloma patients >/= 65 years of age undergoing high-dose therapy and autologous stem cell transplantation: a multicentric observational registry study. Bone Marrow Transplant. 2018. e-pub ahead of print 2018/11/06; 10.1038/s41409-018-0379-y
Garderet L, Beohou E, Caillot D, Stoppa AM, Touzeau C, Chretien ML, et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica. 2016;101:1390–7. e-pub ahead of print 2016/11/02
Badros A, Barlogie B, Siegel E, Morris C, Desikan R, Zangari M, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–7. e-pub ahead of print 2001/09/13
Acknowledgements
We thank Ms A Nakamura for her valuable secretarial assistance and all of the physicians and staff members of the collaborating institutes of the Japan Society for Hematopoietic Stem Cell Transplantation.
Funding
This work was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from Japan Agency for Medical Research and Development, AMED under Grant Number 18ek0510023h0002.
Author contributions
SM designed the research and wrote the first draft of the manuscript. SM and KK analyzed the data and performed the statistical analysis. KK, IH, KS, HT, and AT contributed to the critical review of the manuscript. All the other authors contributed to data collection. All authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SM received honoraria from Takeda Pharmaceutical Co., Ltd. and received research funding from Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd, and Bristol-Myers Squibb Corporation. IH received lecture fee from Celgene Corporation, Bristol-Myers Squibb Corporation, Janssen Corporation, Takeda Pharmaceutical Co., Ltd, and Eisai Co., Ltd, and received research funding from Fujimoto Pharmaceutical Corporation, Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd, and Bristol-Myers Squibb. KS received honoraria from Ono Pharmaceutical Co., Ltd, Bristol-Myers Squibb Corporation, and Celgene Corporation, and received research funding from Ono Pharmaceutical Co., Ltd, Bristol-Myers Squibb Corporation, Celgene Corporation, Janssen Corporation, Takeda Pharmaceutical Co., Ltd, Sanofi Corporation, Abbvie Corporation, GlaxoSmithKline plc, MSD Corporation, and Daiichi Sankyo Co., Ltd. HT received honoraria from Celgene Corporation and Janssen Corporation, and received research funding from Ono Pharmaceutical Co., Ltd, Bristol-Myers Squibb, and Celgene Corporation. AT received research funding from Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd, and Bristol-Myers Squibb Corporation.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mizuno, S., Kawamura, K., Hanamura, I. et al. Efficacy and safety of autologous stem cell transplantation in patients aged ≥ 65 years with multiple myeloma in the era of novel agents. Bone Marrow Transplant 54, 1595–1604 (2019). https://doi.org/10.1038/s41409-019-0478-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0478-4
This article is cited by
-
Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents
Bone Marrow Transplantation (2021)
-
Autologous stem cell transplantation in elderly patients with multiple myeloma in Korea: the KMM1807 study
International Journal of Hematology (2020)