Abstract
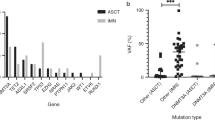
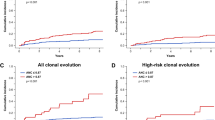
Age-related somatic mutations linked to clonal hematopoiesis have been found in apparently healthy individuals and increase the risk of developing hematologic malignancies. In acute myeloid leukemia (AML) the clinical relevance of clonal hematopoiesis remains controversial and data on patients with detectable clonal hematopoiesis, consolidated with hematopoietic stem cell transplantation are limited. We analyzed samples from 113 AML patients in complete remission prior to hematopoietic stem cell transplantation for the presence of clonal hematopoiesis-associated mutations. The results were correlated with clinical and biological data. In complete remission we found 75 mutations previously linked to clonal hematopoiesis in 47 patients (41.6%). Twenty patients had ≥2 mutations linked to clonal hematopoiesis. DNMT3A, TET2, and ASXL1 were most frequently mutated. When compared to pre-treatment samples we found variable patterns of mutation persistence depending on the gene mutated. In AML patients after allogeneic hematopoietic stem cell transplantation the presence of clonal hematopoiesis-associated mutations in complete remission did not associate with inferior clinical outcome. This study demonstrates that clonal hematopoiesis is a frequent phenomenon in AML patients. Presence of clonal hematopoiesis has no negative prognostic impact in the context of an allogeneic hematopoietic stem cell transplantation and might be beneficial if certain genes are affected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8. https://doi.org/10.1038/nm.3733
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. https://doi.org/10.1056/NEJMoa1408617
McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10:1239–45. https://doi.org/10.1016/j.celrep.2015.02.005
Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484 https://doi.org/10.1038/ncomms12484
Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87. https://doi.org/10.1056/NEJMoa1409405
Buscarlet M, Provost S, Feroz Zada Y, Barhdadi A, Bourgoin V, Lépine G, et al. DNMT3A and TET2 dominate clonal hematopoiesis, demonstrate benign phenotypes and different genetic predisposition. Blood. 2017; https://doi.org/10.1182/blood-2017-04-777029
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. https://doi.org/10.1182/blood-2015-03-631747
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21. https://doi.org/10.1056/NEJMoa1701719
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33. https://doi.org/10.1038/nature13038
Corces-Zimmerman MR, Hong W-J, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. 2014;111:2548–53. https://doi.org/10.1073/pnas.1324297111
Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–605. https://doi.org/10.1200/JCO.2016.71.6712
Bhatnagar B, Eisfeld A-K, Nicolet D, Mrozek K, Blachly JS, Orwick S, et al. Persistence of DNMT3A R882 mutations during remission does not adversely affect outcomes of patients with acute myeloid leukaemia. Br J Haematol. 2016;175:226–36. https://doi.org/10.1111/bjh.14254
Gaidzik VI, Weber D, Paschka P, Kaumanns A, Krieger S, Corbacioglu A, et al. DNMT3A mutant transcript levels persist in remission and do not predict outcome in patients with acute myeloid leukemia. Leukemia. 2017; https://doi.org/10.1038/leu.2017.200
Ploen GG, Nederby L, Guldberg P, Hansen M, Ebbesen LH, Jensen UB, et al. Persistence of DNMT3A mutations at long-term remission in adult patients with AML. Br J Haematol. 2014;167:478–86. https://doi.org/10.1111/bjh.13062
Rothenberg-Thurley M, Amler S, Goerlich D, Kohnke T, Konstandin NP, Schneider S, et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2018;32:1598–608. https://doi.org/10.1038/s41375-018-0034-z
Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378:1189–99. https://doi.org/10.1056/NEJMoa1716863
Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36:1788–97. https://doi.org/10.1200/JCO.2017.77.6757
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. https://doi.org/10.1182/blood-2016-08-733196
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620 https://doi.org/10.1182/blood-2002-05-1340
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390 https://doi.org/10.1182/blood.V97.11.3390
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–67. https://doi.org/10.1200/JCO.2009.27.1460
Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–53. https://doi.org/10.1200/JCO.2005.03.1765
Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrozek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN favorable genetic category. Blood. 2011;118:6920–9. https://doi.org/10.1182/blood-2011-08-368225
Lin J, Yang J, Wen X-M, Yang L, Deng Z-Q, Qian Z, et al. Detection of SRSF2-P95 mutation by high-resolution melting curve analysis and its effect on prognosis in myelodysplastic syndrome. PLoS ONE. 2014;9:e115693 https://doi.org/10.1371/journal.pone.0115693
Bill M, Jentzsch M, Grimm J, Schubert K, Lange T, Cross M, et al. Prognostic impact of the European LeukemiaNet standardized reporting system in older AML patients receiving stem cell transplantation after non-myeloablative conditioning. Bone Marrow Transplant. 2017;52:932–5. https://doi.org/10.1038/bmt.2017.42
Jentzsch M, Bill M, Nicolet D, Leiblein S, Schubert K, Pless M, et al. Prognostic impact of the CD34+/CD38− cell burden in patients with acute myeloid leukemia receiving allogeneic stem cell transplantation. Am J Hematol. 2017;92:388–96. https://doi.org/10.1002/ajh.24663
Benthaus T, Schneider F, Mellert G, Zellmeier E, Schneider S, Kakadia PM, et al. Rapid and sensitive screening for CEBPA mutations in acute myeloid leukaemia. Br J Haematol. 2008;143:230–9. https://doi.org/10.1111/j.1365-2141.2008.07328.x
Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients age 70 years and older in the United States. Blood. 2017; https://doi.org/10.1182/blood-2017-03-772368
Hirsch P, Tang R, Abermil N, Flandrin P, Moatti H, Favale F, et al. Precision and prognostic value of clone-specific minimal residual disease in acute myeloid leukemia. Haematologica. 2017;102:1227–37. https://doi.org/10.3324/haematol.2016.159681
Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA. 2015;314:811–22. https://doi.org/10.1001/jama.2015.9643
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2010;29:789–96. https://doi.org/10.1200/JCO.2010.32.8021
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. https://doi.org/10.1056/NEJMoa1002028
Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–55. https://doi.org/10.1200/JCO.2009.27.3730
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. https://doi.org/10.1056/NEJMoa1516192
Wong TN, Miller CA, Klco JM, Petti A, Demeter R, Helton NM, et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood. 2016;127:893–7. https://doi.org/10.1182/blood-2015-10-677021
Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–62. https://doi.org/10.1182/blood-2014-04-567933
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. https://doi.org/10.1126/science.aaa4971
Strønen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science. 2016;352:1337–41. https://doi.org/10.1126/science.aaf2288
Acknowledgements
This work was supported by the Deutsche José-Carreras-Stiftung (#04R/2016 and #PS15/05 J.G.), Verein zusammen gegen den Krebs e.V., and Ein Herz für Kinder e.V. The authors thank Janet Bogardt, Annette Jilo, Dagmar Cron, Ines Kovacs, Kathrin Wildenberger, Scarlett Schwabe, Christine Günther, Daniela Bretschneider, Evelin Hennig, and Christel Müller for their assistance. The authors like to acknowledge Prof. Ralf Burckhardt and Dr. Max Hubmann for support in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Grimm, J., Bill, M., Jentzsch, M. et al. Clinical impact of clonal hematopoiesis in acute myeloid leukemia patients receiving allogeneic transplantation. Bone Marrow Transplant 54, 1189–1197 (2019). https://doi.org/10.1038/s41409-018-0413-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0413-0
This article is cited by
-
Prognostic impact of the AML ELN2022 risk classification in patients undergoing allogeneic stem cell transplantation
Blood Cancer Journal (2022)
-
ELN risk stratification and outcomes in secondary and therapy-related AML patients consolidated with allogeneic stem cell transplantation
Bone Marrow Transplantation (2021)
-
Prognostic value of measurable residual disease monitoring by next-generation sequencing before and after allogeneic hematopoietic cell transplantation in acute myeloid leukemia
Blood Cancer Journal (2021)
-
Clonal hematopoiesis of indeterminate potential in older patients having received an allogeneic stem cell transplantation from young donors
Bone Marrow Transplantation (2020)
-
Allogeneic stem cell transplantation mitigates the adverse prognostic impact of high diagnostic BAALC and MN1 expression in AML
Annals of Hematology (2020)