Abstract
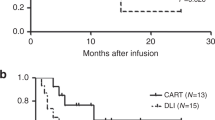
The source of CAR T cells can be autologous (autoCAR) or allogeneic (alloCAR). The latter is seen in patients with a history of allogeneic hematopoietic stem cell transplantation, and can be either donor-derived (DD-alloCAR) or recipient-derived (RD-alloCAR). While autoCAR is activated by CAR only, alloCAR receives activation signals from both T-cell receptor (TCR) and CAR. As a result, the biological differences could impact clinical outcomes. We retrospectively reviewed 31 patients: 17 received autoCAR, 11 received RD-alloCAR, and 3 received DD-alloCAR. After a median follow-up of 9 months, CR rate was 88.2% (95% CI 63.6–98.5%) in autoCAR and 100% (95% CI 71.5–100%) in RD-alloCAR. The median peak expansion in the autoCAR was significantly higher than the RD-alloCAR group (p = 0.007). RD-alloCAR group had significantly less patients with severe CRS (Grade ≥ 3) than the autoCAR group (p = 0.049). Acute graft-versus-host disease (GVHD) occurred in 2 (18.2%) of RD-alloCAR patients and 1 (33.3%) of DD-alloCAR patients. Univariate subgroup analysis of alloCAR group showed the presence of cGVHD at the time of T-cell collection was significantly associated with less than 6-month relapses (p = 0.022). RD-alloCAR patients with or without cGVHD at PBMC collection did not differ regarding the peak CAR T-cell expansion, CRS grades and OS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood. 2018;131:1045–52.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
Collins RH, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–6.
Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–87.
Yang Y, Jacoby E, Fry TJ. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr Opin Hematol. 2015;22:509–15.
Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2016;37:220–30.
Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9:eaag1209.
Bridgeman JS, Ladell K, Sheard VE, Miners K, Hawkins RE, Price DA, et al. CD3ζ-based chimeric antigen receptors mediate T cell activation via cis- and trans-signalling mechanisms: implications for optimization of receptor structure for adoptive cell therapy. Clin Exp Immunol. 2014;175:258–67.
Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–73.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225
Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4:e1027469.
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126:3363–76.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-Cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, et al. Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017;23:3297–306.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e381.
Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain MCAR. T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8.
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48.
Hainz U, Obexer P, Winkler C, Sedlmayr P, Takikawa O, Greinix H, et al. Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation. Blood. 2005;105:4127–34.
Bachireddy P, Wu CJ. Understanding anti-leukemia responses to donor lymphocyte infusion. Oncoimmunology. 2014;3:e28187.
Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–9.
Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The bbiology of cchronic graft-versus-host disease: a task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–34.
Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–80.
Signori A, Crocchiolo R, Oneto R, Sacchi N, Sormani MP, Fagioli F, et al. Chronic GVHD is associated with lower relapse risk irrespective of stem cell source among patients receiving transplantation from unrelated donors. Bone Marrow Transplant. 2012;47:1474–8.
Acknowledgements
We thank Qu Cui, PhD for the editorial assistance.
Funding
This work was supported by the grants from 973 Program (2015CB964900), the Natural Science Foundation of China (81470341, 81770201, 81730008), Key Project of Science and Technology Department of Zhejiang Province (2018C03016-2, 2015C03G2150011), Zhejiang public welfare foundation (GF18H180002).
Author contributions
H.H. designed the study. Y.H. and J.W. were responsible for writing the manuscript. J.W. and J.Y. collected and analyzed all data. G.W., Y.L., J.S., W.W., K.Z., L.X., Y.Z., Z.W., H.X., and A.H.C. participated in the study. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted according to the ethical principles for medical research involving human subjects as stated in the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Boards of the First Affiliated Hospital, School of Medicine, Zhejiang University. All participants signed informed consent.
Rights and permissions
About this article
Cite this article
Hu, Y., Wang, J., Wei, G. et al. A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplant 54, 1208–1217 (2019). https://doi.org/10.1038/s41409-018-0403-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0403-2
This article is cited by
-
Deciphering and advancing CAR T-cell therapy with single-cell sequencing technologies
Molecular Cancer (2023)
-
Chimeric Antigen Receptor CAR-T Therapy on the Move: Current Applications and Future Possibilities
Current Tissue Microenvironment Reports (2023)
-
Alliance between titans: combination strategies of CAR-T cell therapy and oncolytic virus for the treatment of hematological malignancies
Annals of Hematology (2023)
-
Strategies to enhance CAR-T persistence
Biomarker Research (2022)
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)