Abstract
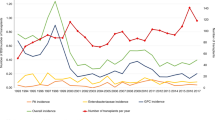
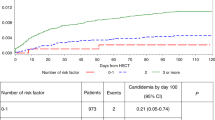
We analyzed CIBMTR data to evaluate the incidence of non-relapse mortality (NRM) and association with overall survival (OS) for bacterial blood stream infections (BSIs) occurring within 100 days of alloHCT in 2 different phases: pre-/peri-engraftment (BSI very early phase, BSI-VEP) and BSI post-engraftment (BSI occurring between 2 weeks after engraftment and day 100, late early phase, BSI-LEP). Of the 7128 alloHCT patients, 2656 (37%) had ≥1 BSI by day 100. BSI-VEP, BSI-LEP, and BSI-Both constituted 56% (n = 1492), 31% (n = 824), and 13% (n = 340) of total BSI, respectively. Starting in 2009, we observed a gradual decline in BSI incidence through 2012 (61–48%). Patients with BSI-VEP were more likely to receive a myeloablative conditioning (MAC) regimen with total body irradiation (TBI). NRM was significantly higher in patients with any BSI (RR 1.82 95% CI 1.63–2.04 for BSI-VEP, RR 2.46, 95% CI 2.05–2.96 for BSI-LEP, and RR 2.29, 95% CI 1.87–2.81 for BSI-Both) compared with those without BSI. OS was significantly lower in patients with any BSI compared with patients without BSI (RR 1.36, 95% CI 1.26–1.47 for BSI-VEP; RR 1.83, 95% CI 1.58–2.12 for BSI-LEP: RR 1.66, 95% CI 1.43–1.94 for BSI-Both). BSIs within day 100 after alloHCT are common and remain a risk factor for mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Meyer E, Beyersmann J, Bertz H, Wenzler-Rottele S, Babikir R, Schumacher M, et al. Risk factor analysis of blood stream infection and pneumonia in neutropenic patients after peripheral blood stem-cell transplantation. Bone Marrow Transplant. 2007;39:173–8.
Hong J, Moon SM, Ahn HK, Sym SJ, Park YS, Park J, et al. Comparison of characteristics of bacterial bloodstream infection between adult patients with allogeneic and autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:994–9.
Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15:47–53.
Oliveira AL, de Souza M, Carvalho-Dias VM, Ruiz MA, Silla L, Tanaka PY, et al. Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;39:775–81.
Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR. Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant. 2011;46:300–7.
Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M. Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis. 2014;16:106–14.
Young JA, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22:359–70.
See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol. 2013;34:769–76.
The Centers for Disease Control and Prevention (CDC) Manuel. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection). 2016. http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf
Ballen K, Ahn KW, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1636–45.
Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70.
Kim SH, Kee SY, Lee DG, Choi SM, Park SH, Kwon JC, et al. Infectious complications following allogeneic stem cell transplantation: reduced-intensity vs. myeloablative conditioning regimens. Transpl Infect Dis. 2013;15:49–59.
Mitchell AE, Derrington P, Turner P, Hunt LP, Oakhill A, Marks DI. Gram-negative bacteraemia (GNB) after 428 unrelated donor bone marrow transplants (UD-BMT): risk factors, prophylaxis, therapy and outcome. Bone Marrow Transplant. 2004;33:303–10.
Dandoy CE, Haslam D, Lane A, Jodele S, Demmel K, El-Bietar J, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1671–7.
Orasch C, Weisser M, Mertz D, Conen A, Heim D, Christen S, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:521–6.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389.e1–401.e1.
Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1:145–56.
Kikuchi M, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17:56–65.
Gudiol C, Garcia-Vidal C, Arnan M, Sanchez-Ortega I, Patino B, Duarte R, et al. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49:824–30.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96.
Ustun C, Arslan O, Beksac M, Koc H, Gurman G, Ozcelik T, et al. A retrospective comparison of allogeneic peripheral blood stem cell and bone marrow transplantation results from a single center: a focus on the incidence of graft-vs.-host disease and relapse. Biol Blood Marrow Transplant. 1999;5:28–35.
Mihu CN, Schaub J, Kesh S, Jakubowski A, Sepkowitz K, Pamer EG, et al. Risk factors for late Staphylococcus aureus bacteremia after allogeneic hematopoietic stem cell transplantation: a single-institution, nested case-controlled study. Biol Blood Marrow Transplant. 2008;14:1429–33.
Wang CH, Chang FY, Chao TY, Kao WY, Ho CL, Chen YC, et al. Characteristics comparisons of bacteremia in allogeneic and autologous hematopoietic stem cell-transplant recipients with levofloxacin prophylaxis and influence on resistant bacteria emergence. J Microbiol Immunol Infect. 2018;51:123–31.
Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7:11–7.
Remberger M, Ackefors M, Berglund S, Blennow O, Dahllof G, Dlugosz A, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17:1688–97.
Ueda M, Berger M, Gale RP, Lazarus HM. Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev. 2018;32:106–15.
Ozcan M, Ustun C, Akcaglayan E, Akan H, Arslan O, Ilhan O, et al. Recombinant human granulocyte colony-stimulating factor (rh-G-CSF) may accelerate hematopoietic recovery after HLA-identical sibling allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;27:499–505.
Engelhard D, Akova M, Boeckh MJ, Freifeld A, Sepkowitz K, Viscoli C, et al. Bacterial infection prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44:467–70.
Riches M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface. Bone Marrow Transplant. 2009;44:453–5.
Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8:339ra371.
CIBMTR Support List
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
CIBMTR Support List: The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ustun, C., Young, JA.H., Papanicolaou, G.A. et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 54, 1254–1265 (2019). https://doi.org/10.1038/s41409-018-0401-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0401-4
This article is cited by
-
Post-transplantation cyclophosphamide is associated with increased bacterial infections
Bone Marrow Transplantation (2024)
-
Impact of intensified contact precautions while treating hematopoietic stem cell transplantation recipients during aplasia
European Journal of Medical Research (2023)
-
Bacterial Sequencing Reads in Blood Exome Files from Melanoma and Cervical Cancer Patients are Associated with Cancer Recurrence
Molecular Biotechnology (2023)
-
Supporting the gastrointestinal microenvironment during high-dose chemotherapy and stem cell transplantation by inhibiting IL-1 signaling with anakinra
Scientific Reports (2022)
-
Grading bloodstream infection risk using citrulline as a biomarker of intestinal mucositis in patients receiving intensive therapy
Bone Marrow Transplantation (2022)