Abstract
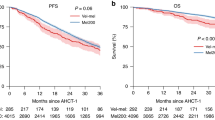
The optimal melphalan dose prior to autologous stem cell transplantation (ASCT) is not known for elderly multiple myeloma (MM) patients. We analyzed data of all MM patients ≥65 years (n = 388) enrolled in the observational Swiss Blood Stem Cell Transplantation Registry. The median age was 67 years (65–77). Single ASCT was performed in 344 (88.7%) patients, with 259 patients (75.3%) receiving a melphalan dose of 200 mg/m2 (MEL200), and 85 patients (24.7%) receiving lower doses (MELlow) (median 140 mg/m2, range 70−180 mg/m2). MEL200 patients were slightly younger, and had a better renal function, but did not differ with regards to ISS stage, cytogenetic risk, remission status, and KPS. Overall mortality at day 100 was 1.5% without differences between the MEL groups (p = 0.621). Median progression-free survival (PFS) in the MEL200 and the MELlow group was 27.7 and 22.1 months, respectively (p = 0.294). Median overall survival (OS) in the MEL200 and in MELlow group was 91.2 and 61.2 months (p = 0.015). However, multivariate analysis showed no significant association of the melphalan dose and OS (HR 0.734; CI95% 0.264–2.038; p = 0.553). In conclusion, our data reveal no significant differences in safety and PFS for elderly myeloma patients treated with MEL200 or with lower MEL doses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. https://doi.org/10.1056/NEJMoa022340
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. https://doi.org/10.1056/NEJMoa1402888
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20. https://doi.org/10.1056/NEJMoa1611750
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9. https://doi.org/10.1200/JCO.2015.61.2267
Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M, et al. Melphalan 200 mg/m(2) versus melphalan 100 mg/m(2) in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. 2010;115:1873–9. https://doi.org/10.1182/blood-2009-09-241737
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
Jantunen E. Autologous stem cell transplantation beyond 60 years of age. Bone Marrow Transplant. 2006;38:715–20. https://doi.org/10.1038/sj.bmt.1705514
Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. 2014;32:2531–40. https://doi.org/10.1200/JCO.2014.55.1028
Badros A, Barlogie B, Siegel E, Morris C, Desikan R, Zangari M, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–7.
Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S, Leung N, et al. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2007;39:605–11. https://doi.org/10.1038/sj.bmt.1705627
Jantunen E, Kuittinen T, Penttila K, Lehtonen P, Mahlamaki E, Nousiainen T. High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (> or =65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone Marrow Transplant. 2006;37:917–22. https://doi.org/10.1038/sj.bmt.1705360
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–7. https://doi.org/10.1182/blood-2004-02-0408
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–18. https://doi.org/10.1016/S0140-6736(07)61537-2
Auner HW, Szydlo R, Hoek J, Goldschmidt H, Stoppa AM, Morgan GJ, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transplant. 2015;50:209–15. https://doi.org/10.1038/bmt.2014.255
Ozaki S, Harada T, Saitoh T, Shimazaki C, Itagaki M, Asaoku H, et al. Survival of multiple myeloma patients aged 65-70 years in the era of novel agents and autologous stem cell transplantation. A multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014;132:211–9. https://doi.org/10.1159/000357394
Sharma M, Zhang MJ, Zhong X, Abidi MH, Akpek G, Bacher U, et al. Older patients with myeloma derive similar benefit from autologous transplantation. Biol Blood Marrow Transplant. 2014;20:1796–803. https://doi.org/10.1016/j.bbmt.2014.07.013
Auner HW, Garderet L, Kroger N. Autologous haematopoietic cell transplantation in elderly patients with multiple myeloma. Br J Haematol. 2015;171:453–62. https://doi.org/10.1111/bjh.13608
Palumbo A, Triolo S, Baldini L, Callea V, Capaldi A, De Stefano V, et al. Dose-intensive melphalan with stem cell support (CM regimen) is effective and well tolerated in elderly myeloma patients. Haematologica. 2000;85:508–13.
Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999;93:51–54.
Sirohi B, Powles R, Treleaven J, Mainwaring P, Kulkarni S, Pandha H, et al. The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone Marrow Transplant. 2000;25:533–9. https://doi.org/10.1038/sj.bmt.1702188
Merz M, Neben K, Raab MS, Sauer S, Egerer G, Hundemer M, et al. Autologous stem cell transplantation for elderly patients with newly diagnosed multiple myeloma in the era of novel agents. Ann Oncol. 2014;25:189–95. https://doi.org/10.1093/annonc/mdt509
Wildes TM, Finney JD, Fiala M, Gao F, Vij R, Stockerl-Goldstein K, et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant. 2015;50:1075–82. https://doi.org/10.1038/bmt.2015.106
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20. https://doi.org/10.1200/JCO.2005.04.242
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. https://doi.org/10.1038/sj.leu.2404284
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46. https://doi.org/10.1016/S1470-2045(16)30206-6
Garderet L, Beohou E, Caillot D, Stoppa AM, Touzeau C, Chretien ML, et al. Upfront autologous stem cell transplantation for newly diagnosed elderly multiple myeloma patients: a prospective multicenter study. Haematologica. 2016;101:1390–7. https://doi.org/10.3324/haematol.2016.150334
Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH, et al. Melphalan 140 mg/m(2) or 200 mg/m(2) for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica. 2018;103:514–21. https://doi.org/10.3324/haematol.2017.181339
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. https://doi.org/10.1056/NEJMoa032290
Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Blade J, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–73. https://doi.org/10.1182/blood-2011-02-297325
Kleber M, Ihorst G, Terhorst M, Koch B, Deschler B, Wasch R, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1:e35 https://doi.org/10.1038/bcj.2011.34
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74. https://doi.org/10.1182/blood-2014-12-615187
Engelhardt M, Domm AS, Dold SM, Ihorst G, Reinhardt H, Zober A, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–21. https://doi.org/10.3324/haematol.2016.162693
Acknowledgements
This work was supported by a grant from ABREOC 2016 to BG.
Author information
Authors and Affiliations
Consortia
Contributions
BG, GG and MK designed the study, analyzed the data, and wrote the first draft of the manuscript; GG and GS performed statistical analyses; all authors collected patient data, and critically read, discussed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This work has been presented at the 59th Annual Meeting of the American Society of Hematology, 2017.
Rights and permissions
About this article
Cite this article
Ghilardi, G., Pabst, T., Jeker, B. et al. Melphalan dose in myeloma patients ≥65 years of age undergoing high-dose therapy and autologous stem cell transplantation: a multicentric observational registry study. Bone Marrow Transplant 54, 1029–1037 (2019). https://doi.org/10.1038/s41409-018-0379-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0379-y
This article is cited by
-
Clinical factors associated with autologous stem cell transplantation outcomes in multiple myeloma: upfront transplant with MEL200 remains the standard of care
Annals of Hematology (2024)
-
Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents
Bone Marrow Transplantation (2021)
-
Melphalan 200 mg/m2 does not increase toxicity and improves survival in comparison to reduced doses of melphalan in multiple myeloma patients
Bone Marrow Transplantation (2021)
-
Efficacy and safety of autologous stem cell transplantation in patients aged ≥ 65 years with multiple myeloma in the era of novel agents
Bone Marrow Transplantation (2019)