Abstract
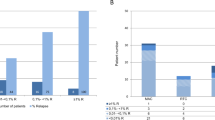
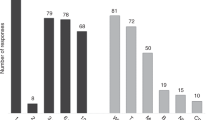
Conventional analysis of host chimerism (HC) frequently fails to detect relapse before its clinical manifestation in patients with hematological malignancies after allogeneic stem cell transplantation (allo-SCT). Quantitative PCR (qPCR)-based highly-sensitive chimerism analysis extends the detection limit of conventional (short tandem repeats-based) chimerism analysis from 1 to 0.01% host cells in whole blood. To date, the diagnostic value of highly-sensitive chimerism analysis is hardly defined. Here, we applied qPCR-based chimerism analysis to 901 blood samples of 71 out-patients with hematological malignancies after allo-SCT. Receiver operating characteristics (ROC) curves were calculated for absolute HC values and for the increments of HC before relapse. Using the best cut-offs, relapse was detected with sensitivities of 74 or 85% and specificities of 69 or 75%, respectively. Positive predictive values (PPVs) were only 12 or 18%, but the respective negative predictive values were 98 or 99%. Relapse was detected median 38 or 45 days prior to clinical diagnosis, respectively. Considering also durations of steadily increasing HC of more than 28 days improved PPVs to more than 28 or 59%, respectively. Overall, highly-sensitive chimerism analysis excludes relapses with high certainty and predicts relapses with high sensitivity and specificity more than a month prior to clinical diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rubio MT, Savani BN, Labopin M, Polge E, Niederwieser D, Ganser A, et al. The impact of HLA-matching on reduced intensity conditioning regimen unrelated donor allogeneic stem cell transplantation for acute myeloid leukemia in patients above 50 years-a report from the EBMT acute leukemia working party. J Hematol & Oncol. 2016;9:65 https://doi.org/10.1186/s13045-016-0295-9.
Koenecke C, Gohring G, de Wreede LC, van Biezen A, Scheid C, Volin L, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic syndromes: a retrospective multicenter study of the European Society of Blood and Marrow Transplantation. Haematologica. 2015;100:400–8. https://doi.org/10.3324/haematol.2014.116715.
Nishiwaki S, Inamoto Y, Sakamaki H, Kurokawa M, Iida H, Ogawa H, et al. Allogeneic stem cell transplantation for adult Philadelphia chromosome-negative acute lymphocytic leukemia: comparable survival rates but different risk factors between related and unrelated transplantation in first complete remission. Blood. 2010;116:4368–75. https://doi.org/10.1182/blood-2010-02-269571.
Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions—a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2015;21:653–60. https://doi.org/10.1016/j.bbmt.2014.12.016.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–8. https://doi.org/10.1182/blood-2011-04-348805.
Kroger N, Bacher U, Bader P, Bottcher S, Borowitz MJ, Dreger P, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2010;16:1187–211. https://doi.org/10.1016/j.bbmt.2010.06.008
Kroger N, Bacher U, Bader P, Bottcher S, Borowitz MJ, Dreger P, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on disease-specific methods and strategies for monitoring relapse following allogeneic stem cell transplantation. Part II: chronic leukemias, myeloproliferative neoplasms, and lymphoid malignancies. Biol Blood Marrow Transplant. 2010;16:1325–46. https://doi.org/10.1016/j.bbmt.2010.07.001.
Radich JP, Gehly G, Gooley T, Bryant E, Clift RA, Collins S, et al. Polymerase chain reaction detection of the BCR-ABL fusion transcript after allogeneic marrow transplantation for chronic myeloid leukemia: results and implications in 346 patients. Blood. 1995;85:2632–8.
Gorello P, Cazzaniga G, Alberti F, Dell’Oro MG, Gottardi E, Specchia G, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20:1103–8. https://doi.org/10.1038/sj.leu.2404149.
Beretta C, Gaipa G, Rossi V, Bernasconi S, Spinelli O, Dell’Oro MG, et al. Development of a quantitative-PCR method for specific FLT3/ITD monitoring in acute myeloid leukemia. Leukemia. 2004;18:1441–4. https://doi.org/10.1038/sj.leu.2403409.
Barrios M, Jimenez-Velasco A, Roman-Gomez J, Madrigal ME, Castillejo JA, Torres A, et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica. 2003;88:801–10.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–705. https://doi.org/10.1200/jco.2004.05.198.
Frankel W, Chan A, Corringham RE, Shepherd S, Rearden A, Wang-Rodriguez J. Detection of chimerism and early engraftment after allogeneic peripheral blood stem cell or bone marrow transplantation by short tandem repeats. Am J Hematol. 1996;52:281–7.
Suttorp M, Schmitz N, Dreger P, Schaub J, Loffler H. Monitoring of chimerism after allogeneic bone marrow transplantation with unmanipulated marrow by use of DNA polymorphisms. Leukemia. 1993;7:679–87.
Choi SJ, Lee KH, Lee JH, Kim S, Chung HJ, Lee JS, et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 2000;26:327–32. https://doi.org/10.1038/sj.bmt.1702504.
Bornhauser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34 + peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94:1613–7. https://doi.org/10.3324/haematol.2009.007765.
Hoffmann JC, Stabla K, Burchert A, Volkmann T, Bornhauser M, Thiede C, et al. Monitoring of acute myeloid leukemia patients after allogeneic stem cell transplantation employing semi-automated CD34 + donor cell chimerism analysis. Ann Hematol. 2014;93:279–85. https://doi.org/10.1007/s00277-013-1961-4.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–25.
Maas F, Schaap N, Kolen S, Zoetbrood A, Buno I, Dolstra H, et al. Quantification of donor and recipient hemopoietic cells by real-time PCR of single nucleotide polymorphisms. Leukemia. 2003;17:621–9. https://doi.org/10.1038/sj.leu.2402856.
Jimenez-Velasco A, Barrios M, Roman-Gomez J, Navarro G, Buno I, Castillejo JA, et al. Reliable quantification of hematopoietic chimerism after allogeneic transplantation for acute leukemia using amplification by real-time PCR of null alleles and insertion/deletion polymorphisms. Leukemia. 2005;19:336–43. https://doi.org/10.1038/sj.leu.2403622.
Fehse B, Chukhlovin A, Kuhlcke K, Marinetz O, Vorwig O, Renges H, et al. Real-time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J Hematother Stem Cell Res. 2001;10:419–25. https://doi.org/10.1089/152581601750289028.
Chen DP, Tseng CP, Wang WT, Wang MC, Tsai SH, Sun CF. Real-time biallelic polymorphism-polymerase chain reaction for chimerism monitoring of hematopoietic stem cell transplantation relapsed patients. Clin Chim Acta. 2011;412:625–30. https://doi.org/10.1016/j.cca.2010.12.018.
Jacque N, Nguyen S, Golmard JL, Uzunov M, Garnier A, Leblond V, et al. Chimerism analysis in peripheral blood using indel quantitative real-time PCR is a useful tool to predict post-transplant relapse in acute leukemia. Bone Marrow Transplant. 2015;50:259–65. https://doi.org/10.1038/bmt.2014.254.
Kletzel M, Huang W, Olszewski M, Khan S. Validation of chimerism in pediatric recipients of allogeneic hematopoietic stem cell transplantation (HSCT) a comparison between two methods: real-time PCR (qPCR) vs. variable number tandem repeats PCR (VNTR PCR). Chimerism. 2013;4:1–8. https://doi.org/10.4161/chim.23158.
Stahl T, Bohme MU, Kroger N, Fehse B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp Hematol. 2015;43:462 https://doi.org/10.1016/j.exphem.2015.02.006.
Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–9.
van der Torren CR, van Hensbergen Y, Luther S, Aghai Z, Rychnavska ZS, Slot M, et al. Possible role of minor h antigens in the persistence of donor chimerism after stem cell transplantation; relevance for sustained leukemia remission. PloS One. 2015;10:e0119595 https://doi.org/10.1371/journal.pone.0119595.
Sellar RS, Vargas FA, Henry JY, Verfuerth S, Charrot S, Beaton B, et al. CMV promotes recipient T-cell immunity following reduced-intensity T-cell-depleted HSCT, significantly modulating chimerism status. Blood. 2015;125:731–9. https://doi.org/10.1182/blood-2014-07-589150.
Borchers S, Weissinger EM, Pabst B, Ganzenmueller T, Dammann E, Luther S, et al. Expansion of recipient-derived antiviral T cells may influence donor chimerism after allogeneic stem cell transplantation. Transplant Infect Dis. 2013;15:627–33. https://doi.org/10.1111/tid.12101.
Kim SY, Jeong MH, Park N, Ra E, Park H, Seo SH, et al. Chimerism monitoring after allogeneic hematopoietic stem cell transplantation using quantitative real-time PCR of biallelic insertion/deletion polymorphisms. J Mol Diagn: JMD. 2014;16:679–88. https://doi.org/10.1016/j.jmoldx.2014.06.005.
Acknowledgements
This work was supported by the German Federal Ministry of Education and Research (reference number: 01EO1302). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Lea Sellmann was supported by the Hannover Biomedical Research School (HBRS). We thank all the physicians and nurses at the Hannover Medical School transplant unit and in the outpatient clinic for their dedicated work.
Author contributions
Conceived and designed the experiments: LS, LH, and IB. Performed the experiments: LS, KR, and IB. Analyzed the data: LS, IB, and LH. Collection of materials: LS, KR, and IB. Provision of MRD data: GG. Patient documentation: ED. Wrote the paper: LS, AG, EW, and LH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sellmann, L., Rabe, K., Bünting, I. et al. Diagnostic value of highly-sensitive chimerism analysis after allogeneic stem cell transplantation. Bone Marrow Transplant 53, 1457–1465 (2018). https://doi.org/10.1038/s41409-018-0176-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0176-7