Abstract
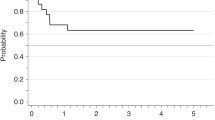
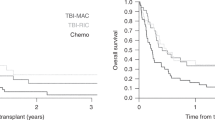
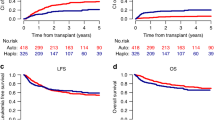
Acute myeloid leukemia with inv(3)(q21;q26.2)/t(3;3)(q21;q26.2) (3q26 AML) is a rare disease with poor prognosis and median survival of <1 year. To evaluate allogeneic stem cell transplantation (alloHSCT) in the treatment of 3q26 AML, we studied 98 patients reported to the European Society for Blood and Marrow Transplantation between 1995 and 2013. Majority of patients were transplanted using peripheral blood, from unrelated donors and after myeloablative conditioning. Fifty-three patients were transplanted with active disease and 45 in complete remission. After a median follow-up of 47 months, 2 year leukemia-free survival (LFS), overall survival (OS), relapse incidence (RI), non-relapse mortality (NRM), and graft-versus-host disease-free, relapse-free survival (GRFS) probabilities were 20%, 26%, 64%, 16%, and 14%, respectively. Two-year LFS and OS probabilities for patients transplanted in CR vs. those transplanted in active disease were 23.8 vs. 17% (p = NS) and 34.9 vs. 18.9% (p = NS), respectively. In multivariate analysis CR was the only factor associated with a trend for better LFS (p = 0.05, HR 0.64) and OS (p = 0.06, HR 0.65). CR also significantly influenced GRFS (p = 0.01; HR 0.55) and NRM (p = 0.02; HR 0.27). The results suggest that a proportion of patients might benefit from the procedure, especially if performed in CR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Swerdlow SH, Campo E, Harris NL, Jaffe SJ, Pileri SA, Stein H, et al. (editors). WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Sun J, Konoplev SN, Wang X, Cui W, Chen SS, Medeiros LJ, et al. De novo acute myeloid leukemia with inv(3)(q21; q26.2) or t(3;3)(q21;q26.2): a clinicopathologic and cytogenetic study of an entity recently added to the WHO classification. Mod Pathol. 2011;24:384–9.
Medeiros BC, Kohrt HE, Arber DA, Bangs CD, Cherry AM, Majeti R, et al. Immunophenotypic featutes of acute myeloid leukemia with inv(3)(q21; q26.2)/t(3;3)(q21;q26.2). Leuk Res. 2010;34:594–7.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council Trials. Blood. 2010;116:354–65.
Lugthart S, Gröschel S, Beverloo HB, Kayser S, Valk PJ, van Zelderen-Bhola SL, et al. Clinical, molecular and prognostic significance of WHO type inv(3)(q21; q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010;28:3890–8.
Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Hospital MA, Thomas X, Castaigne S, Raffoux E, Pautas C, Gardin C, et al. Evaluation of allogeneic hematopoietic SCT in younger adults with adverse karyotype AML. Bone Marrow Transplant. 2012;47:1436–41.
Ferguson P, Hills RK, Grech A, Betterige S, Kjeldsen L, Dennis M, et al. An operational definition of primary refractory acute myeloid leukaemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica. 2016;101:1351–8.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–1.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Poiré X, Labopin M, Maertens J, Yakoub-Agha I, Blaise D, Ifrah N, et al. Allogeneic stem cell transplantation in adult patients with acute myeloid leukaemia and 17p abnormalities in first complete remission: a study from the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2017;18:10–20.
Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–14.
Wanquet A, Prebet T, Berthon C, Sebert M, Roux C, Kulasekararaj A, et al. Azacitidine treatment for patients with myelodysplastic syndrome and acute myeloid leukemia with chromosome 3q abnormalities. Am J Hematol. 2015;90:859–63.
Gröschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BAM, Erpelinck C, et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157:369–81.
Yamazaki H, Suzuki M, Otsuki A, Shimizu R, Bresnick EH, Engel JD, et al. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21; q26) by activating EVI1 expression. Cancer Cell. 2014;25:415–27.
Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34.
Zahler S, Bhatia M, Ricci A, Roy S, Morris E, Harrison L, et al. A phase I study of reduced-intensity conditioning and allogeneic stem cell transplantation followed by dose escalation of targeted consolidation immunotherapy with gemtuzumab ozogamycin in children and adolescents with CD33+ acute myeloid leukemia. Biol Blood Marrow Transplant. 2016;22:698–704.
Terwey TH, Vega-Ruiz A, Hemmati PG, Martus P, Dietz E, le Coutre P, et al. NIH-defined graft-versus-host disease after reduced or myeloablative conditioning in patients with acute myeloid leukemia. Leukemia. 2012;26:536–42.
Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18:1727–33.
Ruggeri A, Battipaglia G, Labopin M, Ehninger G, Beelen D, Tischer J. et al. Unrelated donor versus matched sibling donor in adults with acute myeloid leukemia in first relapse: an ALWP-EBMT study. J Hematol Oncol. 2016;9:89
Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016;51:1431–8.
Savani BN, Labopin M, Blaise D, Niederwieser D, Ciceri F, Ganser A, et al. Peripheral blood stem cell graft compared to bone marrow after reduced intensity conditioning regimens for acute leukemia: a report from the ALWP of the EBMT. Haematologica. 2016;101:256–62.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and Myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
Poiré X, Labopin M, Cornelissen JJ, Volin L, Richard Espiga C, Veelken JH, et al. Outcome of conditioning intensity in acute myeloid leukemia with monosomal karyotype in patients over 45 year-old: a study from the Acute Leukemia Working Party (ALWP) of the European Group of Blood and Marrow Transplantation (EBMT). Am J Hematol. 2015;90:719–24.
Pasquini MC, Zhang M-J, Medeiros BC, Armand P, Hu ZH, Nishihori T, et al. Hematopoietic cell transplantation outcomes in monosomal karyotype myeloid malignancies. Biol Blood Marrow Transplant. 2016;22:248–57.
Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–92.
Rogers HJ, Vardiman JW, Anastasi J, Raca G, Savage NM, Cherry AM, et al. Complex or monosomal karyotype and not blast percentage is associated with poor survival in acute myeloid leukemia and myelodysplastic syndrome patients with inv(3)(q21; q26.6)/t(3;3)(q21;q26.6): A Bone Marrow Pathology Group study. Haematologica. 2014;99:821–8.
Acknowledgements
The authors would like to thank all EBMT centers for their contribution and additional data reporting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Halaburda, K., Labopin, M., Houhou, M. et al. AlloHSCT for inv(3)(q21;q26)/t(3;3)(q21;q26) AML: a report from the acute leukemia working party of the European society for blood and marrow transplantation. Bone Marrow Transplant 53, 683–691 (2018). https://doi.org/10.1038/s41409-018-0165-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0165-x