Abstract
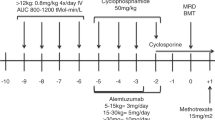
Adult patients with sickle cell disease (SCD) are highly susceptible to stem cell transplant complications, including drug toxicity, graft versus host disease (GVHD), and graft rejection due to SCD-related tissue damage, endothelial activation, and inflammation. The scarcity of compatible stem cells for transplantation further limits treatment options, with only 43 cases of adult allogeneic peripheral blood stem cell transplantation (allo-PSCT) from human leukocyte antigen (HLA)-identical sibling donors reported in the international registry for the period 1986–2013. Herein we report remarkable outcomes in a cohort of adult SCD patients who underwent allo-PSCT using a fludarabine (Flu), busulfan (Bu), and anti-T-cell lymphocyte globulin (ATG)-based conditioning regimen in combination with very low dose total body irradiation (TBI), followed by post-transplant cyclophosphamide (Cy) and sirolimus as GVHD prophylaxis. We performed a single-center, retrospective study consisting of 20 consecutive patients (mean age 33.4 years) who underwent allo-PSCT from HLA-matched related donors with a conditioning regimen of Flu 150/Bu 3.2/Cy 29/ATG 30 (Fresenius)/TBI 200 between September 2013 and September 2017. Data were validated by an independent data audit group of the affiliated JACIE-accredited transplantation center. All patients experienced a sustained donor cell engraftment. Full donor chimerism (total cell) occurred within 180 days in all patients. Mean duration of follow-up was 13.8 months (range: 0.3–50 months), with 12 (60%) patients completing 12 months. No non-relapse mortality or graft rejection occurred. Successful treatment was achieved without the presence of graft loss, grade III–IV acute GVHD, extensive chronic GVHD, or other major complications. Allo-PSCT in combination with Flu 150/Bu 3.2/Cy 29/ATG 30(Fresenius)/TBI 200- Cy/Sirolimus therapy yielded encouraging outcomes with no mortality and low incidence of GVHD. Further controlled studies will be necessary to compare transplant protocols and long-term outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Natarajan K, Townes TM, Kutlar A. Disorders of hemoglobin structure: sickle cell anemia and related abnormalities. In: Kaushansky K, Lichtman MA, Beutler E, Kıpps TJ, Seligsohn U, Prchal JT, editors. Williams hematology, 8th edn. New York: Mcgraw Hill; 2010. p. 667–700.
Maitra P, Caughey M, Robinson L, Desai PC, Jones S, Nouraie M, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102:626–36.
Karacaoglu PK, Asma S, Korur A, Solmaz S, Buyukkurt NT, Gereklioglu C, et al. East Mediterranean region sickle cell disease mortality trial: retrospective multicenter cohort analysis of 735 patients. Ann Hematol. 2016;95:993–1000.
Wierenga KH, Hambleton IR, Lewis NA. Survival estimates for patients with homozygous sickle-cell disease in Jamaica: a clinic-based population study. Lancet. 2001;357:680–3.
Ozdogu H, Sozer O, Boga C, Kozanoglu L, Maytalman E, Guzey M. Flow cytometric evaluation of circulating endothelial cells: a new protocol for identifying endothelial cells at several stages of differentiation. Am J Hematol. 2007;82:706–11.
Sheth S, Licursi M, Bhatia M. Sickle cell disease: time for a closer look at treatment options? Br J Haematol. 2013;162:455–64.
King AA, Kamani N, Bunin N, Sahdev I, Brochstein J, Hayashi RJ, et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90:1093–8.
Matthes-Martin S, Lawitschka A, Fritsch G, Lion T, Grimm B, Breuer S, et al. Stem cell transplantation after reduced-intensity conditioning for sickle cell disease. Eur J Haematol. 2013;90:308–12.
Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129:1548–56.
Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev. 2016;5:CD007001.
Angelucci E, Matthes-Martin S, Baronciani D, Bernaudin F, Bonanomi S, Cappellini MD, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an International Expert Panel. Haematologica. 2014;99:811–20.
Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89:530–5.
Walters MC, De Castro LM, Sullivan KM, De Castro LM, Sullivan KM, Krishnamurti L, et al. Indications and results of HLA-identical sibling hematopoietic cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2016;22:207–11.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811–7.
Fitzhugh CD, Abraham AA, Tisdale J. Hematopoietic stem cell transplantation for patients with sickle cell disease: progress and future directions. Hematol Oncol Clin North Am. 2014;28:1171–85.
Özdoğu H, Boğa C. Hematopoietic stem cell transplantation in adult sickle cell disease: problems and solutions. Turk J Hematol. 2015;32:195–205.
Rotz SJ, O’Riordan MA, Kim C, de Lima M, Gladwin MT, Little JA. Traffic Light: prognosis-based eligibility for clinical trials of hematopoietic SCT in adults with sickle cell anemia. Bone Marrow Transplant. 2015;50:918–23.
Hsieh MM, Fitzhugh CD, Weitzel RP, Link ME, Coles WA, Zhao X, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312:48–56.
Hsieh MM. A standard nonmyeloablative transplantation regimen for adults with sickle cell disease: are we there yet? Biol Blood Marrow Transplant. 2016;22:397–8.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versushost disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–33.
Koçyiğit H, Aydemir Ö, Ölmez N, Memiş A. Kısa Form-36 (KF-36)‘nın Türkçe Versiyonunun Güvenilirliği ve Geçerliliği. İlaç ve Tedavi Derg. 1999;12:102–6.
Ware JE, Kosinski M, Keller S. SF-36 physical and mental health summary scales: a user’s manual. Health Assessment Lab; 1994.
Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):512–21.
Lucarelli G, Isgrò A, Sodani P, Marziali M, Gaziev J, Paciaroni K, et al. Hematopoietic SCT for the Black African and non-Black African variants of sickle cell anemia. Bone Marrow Transplant. 2014;49:1376–81.
Locatelli F, Kabbara N, Ruggeri A, Ghavamzadeh A, Roberts I, Li CK, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–8.
Torres LS, Okumura JV, Silva DG, Mimura KK, Belini-Júnior É, Oliveira RG, et al. Inflammation in sickle cell disease: differential and down-expressed plasma levels of annexin A1 protein. PLoS ONE. 2016;11:e0165833.
Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta thalassemia. Biol Blood Marrow Transplant. 2003;9:519–28.
Bhatia M, Jin Z, Baker C, Geyer MB, Radhakrishnan K, Morris E, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant. 2014;49:913–20.
Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83–89.
Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–11.
Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011;377:1663–72.
Dedeken L, Lê PQ, Azzi N, Brachet C, Heijmans C, Huybrechts S, et al. Haematopoietic stem cell transplantation for severe sickle cell disease in childhood: a single centre experience of 50 patients. Br J Haematol. 2014;165:402–8.
Saraf SL, Oh AL, Patel PR, Jalundhwala Y, Sweiss K, Koshy M, et al. Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol Blood Marrow Transplant. 2016;22:441–8.
Mohty M. Mechanism of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–94.
Mussetti A, Greco R, Peccatori J, Corradini P. Post-transplant cyclophosphamide, a promising anti-graft versus host disease prophylaxis: where do we stand? Expert Rev Hematol. 2017;10:479–92.
Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Posttransplantation cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan-based myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant. 2015;21:1506–14.
Jacoby E, Chen A, Loeb DM, Gamper CJ, Zambidis E, Llosa NJ, et al. Single-agent post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis after human leukocyte antigen-matched related bone marrow transplantation for pediatric and young adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2016;22:112–8.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanskron SM, Gamper CS, Jones RJ, et al. HLA haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–91.
Panch SR, Yau YY, Fitzhugh CD, Hsieh MM, Tisdale JF, Leitman SF. Hematopoietic progenitor cell mobilization is more robust in healthy African American compared to Caucasian donors and is not affected by the presence of sickle cell trait. Transfusion. 2016;56:1058–65.
Gereklioglu C, Asma S, Korur A, Tepebası S, Aytan P, Yeral M, et al. Granulocyte colony stimulated factor administration among hemoglobin S trait donors: a single center experience from the Eastern Meditteranean region. J Clin Apher. 2017. https://doi.org/10.1002/jca.21566.
Arnold SD, Bhatia M, Horan J, Krishnamurti L. Haematopoietic stem cell transplantation for sickle cell disease-current practice and new approaches. Br J Haematol. 2016;174:515–25.
Walters MC, Hardy K, Edwards S, Adamkiewicz T, Barkovich J, Bernaudin F, et al. Multicenter Study of Bone Marrow Transplantation for Sickle Cell Disease. Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16:263–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ozdogu, H., Boga, C., Yeral, M. et al. Allogenic peripheral stem cell transplantation from HLA-matched related donors for adult sickle cell disease: remarkable outcomes from a single-center trial. Bone Marrow Transplant 53, 880–890 (2018). https://doi.org/10.1038/s41409-018-0111-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0111-y
This article is cited by
-
HLA-identical related hematopoietic stem cell transplantation in severe sickle cell disease: age is not a barrier to successful outcome
Bone Marrow Transplantation (2022)
-
Cardiac pathophysiology in sickle cell disease
Journal of Thrombosis and Thrombolysis (2021)
-
Excellent outcomes of allogeneic transplantation from peripheral blood of HLA-matched related donors for adult sickle cell disease with ATLG and posttransplant cyclophosphamide-containing regimen: an update work
Bone Marrow Transplantation (2020)
-
Immunosuppressants
Reactions Weekly (2018)