Abstract
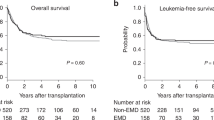
Acute myeloid leukemia (AML) relapse after allogeneic hematopoietic cell transplant (allo-HCT) is challenging. Data on extramedullary relapse (EMR) after allo-HCT are limited. We analyzed 215 patients with AML who underwent allo-HCT in our institution between January 2005 and December 2015. We limited this retrospective review to patients who received a MA conditioning, were in complete remission (CR) at the time of transplant and who received a matched sibling transplant, all other patients were excluded to avoid heterogeneity. Seventy-seven (35.8%) patients experienced disease relapse, 45 had BMR, and 32 had EMR. The only variable that was statistically associated with EMR post allo-HCT was male sex (OR = 3.2 (1.2, 8.2), p-value = 0.01); there was a trend for association between transplant in >CR2 and EMR (OR = 0.38 (0.14, 1.06), p-value = 0.06). The median overall survival (OS) after relapse for all relapses was 10 months (95% CI 4.839–15.161). The median OS for BMR group was 8 months (95% CI 2.850–13.150) and 14 months for the EMR group (95% CI 5.776–22.224); however, this was not statistically significant, p-value = 0.4. Multivariate analysis revealed that gender, treatment modality, and time from allo-HCT to relapse (≥12 vs. <12 months) have significant association with the post-relapse death. Male gender was the only significant factor associated with EMR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
StÃlzel F, Hackmann K, Kuithan F, Mohr B, Fussel M, Oelschlãgel U, et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation. Transplantation. 2012;93:744–9.
Sackstein R. A revision of Billingham’s tenets: the central role of lymphocyte migration in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:2–8.
Jiang YZ, Cullis JO, Kanfer EJ, Goldman JM, Barrett AJ. T cell and NK cell mediated graft-versus-leukaemia reactivity following donor buffy coat transfusion to treat relapse after marrow transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 1993;11:133–8.
Berthou C, Leglise MC, Herry A, Balcon D, Hardy E, Lessard M, et al. Extramedullary relapse after favorable molecular response to donor leukocyte infusions for recurring acute leukemia. Leukemia. 1998;12:676–81.
Au WY, Kwong YL, Lie AK, Ma SK, Liang R. Extra-medullary relapse of leukemia following allogeneic bone marrow transplantation. Hematol Oncol. 1999;17:45–52.
Byrd JC, Weiss RB. Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8; 21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer. 1994;73:2107–12.
Kuwabara H, Nagai M, Yamaoka G, Ohnishi H, Kawakami K. Specific skin manifestations in CD56 positive acute myeloid leukemia. J Cutan Pathol. 1999;26:1–5.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Sullivan KM. Acute and chronic graft-versus-host disease in man. Int J Cell Cloning. 1986;4(S1):42–93.
Harris AC, Kitko CL, Couriel DR, Braun TM, Choi SW, Magenau J, et al. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98:179–84.
Chong G, Byrnes G, Szer J, Grigg A. Extramedullary relapse after allogeneic bone marrow transplantation for haematological malignancy. Bone Marrow Transplant. 2000;26:1011–5.
Lee KH, Lee JH, Choi SJ, Kim S, Seol M, Lee YS, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32:835–42.
Ge L, Ye F, Mao X, Chen J, Sun A, Zhu X, et al. Extramedullary relapse of acute leukemia after allogeneic hematopoietic stem cell transplantation: different characteristics between acute myelogenous leukemia and acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20:1040–7.
Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006;47:1754–67.
Clark WB, Strickland SA, Barrett AJ, Savani BN. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Haematologica. 2010;95:860–3.
Goker H, Gunes G, Demiroglu H, Malkan UY, Haznedaroglu I, Sayinalp N, et al. Extramedullary relapses after allogeneic stem cell transplantation for leukemia: clinical characteristics, cumulative incidence and risk factors. In: ASCO Annual Meeting Proceedings. p. e18005, Chicago, 2015.
Szomor A, Passweg JR, Tichelli A, Hoffmann T, Speck B, Gratwohl A. Myeloid leukemia and myelodysplastic syndrome relapsing as granulocytic sarcoma (chloroma) after allogeneic bone marrow transplantation. Ann Hematol. 1997;75:239–41.
Shimoni A, Rand A, Shem-Tov N, Yerushalmi R, Hardan I, Nagler A. Isolated extra-medullary relapse of acute leukemia after allogeneic stem-cell transplantation (SCT); different kinetics and better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2009;15:59.
Bekassy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;17:801–8.
Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7:50–57.
Simpson DR, Nevill TJ, Shepherd JD, Fung HC, Horsman DE, Nantel SH, et al. High incidence of extramedullary relapse of AML after busulfan/cyclophosphamide conditioning and allogeneic stem cell transplantation. Bone Marrow Transplant. 1998;22:259–64.
Au WY, Ma SK, Kwong YL, Lie AKW, Shek WH, Chow WC, et al. Acute myeloid leukemia relapsing as gynecomastia. Leuk Lymphoma. 1999;36:191–4.
Harris AC, Mageneau J, Braun T, Kitko CL, Choi SW, Ferrara JLM, et al. Extramedullary relapse in acute leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Biol Blood Marrow Transplant. 2013;16:S177–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Alhashim, N., Aljurf, M., Hassanein, M. et al. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia: clinical characteristics, incidence, risk factors and outcomes. Bone Marrow Transplant 53, 838–843 (2018). https://doi.org/10.1038/s41409-018-0093-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0093-9
This article is cited by
-
Myeloid sarcoma: more and less than a distinct entity
Annals of Hematology (2023)
-
Impact of extramedullary disease in AML patients undergoing sequential RIC for HLA-matched transplantation: occurrence, risk factors, relapse patterns, and outcome
Annals of Hematology (2023)
-
Incidence, risk factors and outcome of extramedullary relapse after allogeneic hematopoietic stem cell transplantation in patients with adult acute lymphoblastic leukemia
Annals of Hematology (2020)