Abstract
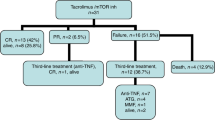
Inhibition of the Janus-associated kinases (JAK) with ruxolitinib (RUX) reduces graft-versus-host disease (GVHD) in preclinical and clinical models. In total 19 allograft recipients with moderate/severe steroid-dependent chronic GVHD received RUX as ≥2nd line salvage. RUX was well tolerated, and led to complete/partial resolution of oral (92/7%), cutaneous (82/0%), hepatic (71/28%), gastro-intestinal (75/17%), musculoskeletal (33/67%), pulmonary (0/80%), scleroderma (0/75%), vaginal (0/75%), and ocular (0/100%) chronic GVHD. Overall 18 achieved partial response and 1 complete response according to NIH Consensus Criteria. Responses occurred early and were sustained which enabled discontinuation (68%) or reduction of steroids to physiologic doses (21%). We conclude that RUX is an effective steroid-sparing agent in chronic GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–84.
Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–9.
Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment?. Hematology. 2012;2012:251–264.
Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. Evaluation of mycofenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–82.
Malik MI, Litzow M, Hogan W, Patnaik M, Murad MH, Prokop LJ, et al. Extracorporeal photopheresis for chronic graft-versus-host disease: a systematic review and metaanalysis. Blood Res. 2014;49:100–6.
Solomon, Sizemore CA, Ridgeway M, Xu Z, Smith J, Brown S, et al. Corticosteroid-free primary treatment of chronic extensive graft-versus-host disease incorporating rituximab. Biol Blood Marrow Transplant. 2015;21:1576–82.
Genovese MC, Kremer J, Zamani O, Ludivico C, Kroguleg M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–52.
Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, et al. IFNyR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120:4093–103.
Choi J, Cooper M, Alahmari B, Ritchey J, Collins L, Holt M, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS ONE 2014;9:e109799.
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogenic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:1–7.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host-disease. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host-disease: IV. The 2014 criteria working group report. Biol Blood Marrow Transplant. 2015;21:984–99.
Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1–17.
Acknowledgements
We thank Dr. Steve Pavletic for his critical review of this manuscript and constructive comments.
Author contributions
HJK and JFD: designed research, performed research, collected data, provided subjects, analyzed and interpreted data, performed statistical analysis, wrote the manuscript; VKK, AAL, IP, AJ, EW, DR, ZAK, IB, and MA performed research, collected data, provided subjects, analyzed and interpreted data, reviewed and approved the manuscript; JW, SB, and ASK: collected data, analyzed and interpreted data, reviewed, and approved the manuscript. JFD is supported by the National Cancer Institute (NIH/NCI: R35 CA210084 and P50 CA171963.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HJK, VKK, EW, and JFD received research support from Incyte, and honoraria for attending advisory board meetings. The remaining authors declare that they have no conflict of interests.
Additional information
Part of this data was presented at the Annual Meeting of the American Society of Hematology in December 2015
Rights and permissions
About this article
Cite this article
Khoury, H.J., Langston, A.A., Kota, V.K. et al. Ruxolitinib: a steroid sparing agent in chronic graft-versus-host disease. Bone Marrow Transplant 53, 826–831 (2018). https://doi.org/10.1038/s41409-017-0081-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-017-0081-5
This article is cited by
-
Propensity score matching analysis comparing the efficacy of Ruxolitinib to historical controls in second-line or beyond treatment for chronic GvHD after steroid failure
Bone Marrow Transplantation (2023)
-
Effect of ruxolitinib on the oral mucosa of patients with steroid-refractory chronic Graft-versus-Host disease and oral involvement
Clinical Oral Investigations (2022)
-
Ruxolitinib in the management of steroid-resistant/-dependent acute and chronic graft-versus-host disease: results of routine practice in an academic centre
Annals of Hematology (2022)
-
Combined treatment of graft versus host disease using donor regulatory T cells and ruxolitinib
Scientific Reports (2022)
-
Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide
Annals of Hematology (2021)