Abstract
The procedure of autologous peripheral blood stem cell transplantation (autoPBSCT) requires cryopreservation of cells in a mixture containing dimethyl sulfoxide (DMSO). DMSO is necessary to secure cell viability, however, its infusion may be toxic to stem cell recipient. The aim of this study was to prospectively evaluate the impact of DMSO concentration on engraftment after autoPBSCT.
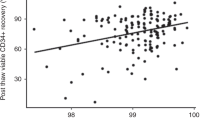
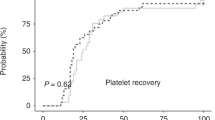
One-hundred-fifty patients were randomly assigned to one of three study arms; their leukapheresis products were cryopreserved in 10%, 7.5% or 5% DMSO. The study groups did not differ with regard to the diagnosis (mainly lymphomas and multiple myeloma), age, conditioning regimen, and the number of transplanted hematopoietic stem cells. 143 patients were treated with autoPBSCT. The frequency of adverse effects during and shortly after infusion was the lowest in 5% DMSO arm (p = 0.02 compared to 10% DMSO). 4 patients died due to infection before the engraftment. The median time to leukocyte and neutrophil recovery was 10 days in all study groups (p = 0.36 and p = 0.2). As well, the median day of platelet recovery was the same for all DMSO concentrations and equaled 15 days (p = 0.61).
In view of these results, 5% DMSO mixture may be considered a new standard in cryopreservation of hematopoietic stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Best BP. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res. 2015;18:422–36.
Donmez A, Tombuloglu M, Gungor A, Soyer N, Saydam G, Cagirgan S. Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci. 2007;36:95–101.
Pamphilon D, Mijovic A. Storage of hemopoietic stem cells. Asian J Transfus Sci. 2007;1:71–76.
Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469–76.
Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T, EBMT Chronic Leukaemia Working Party Complications Subcommittee. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant. 2005;36:601–3.
Morris C, de Wreede L, Scholten M, Brand R, van Biezen A, Sureda A, et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion. 2014;54:2514–22.
Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Glück S. The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004;10:135–41.
Akkök CA, Holte MR, Tangen JM, Ostenstad B, Bruserud O. Hematopoietic engraftment of dimethyl sulfoxide-depleted autologous peripheral blood progenitor cells. Transfusion. 2009;49:354–61.
Lecchi L, Giovanelli S, Gagliardi B, Pezzali I, Ratti I, Marconi M. An update on methods for cryopreservation and thawing of hemopoietic stem cells. Transfus Apher Sci. 2016;54:324–36.
Alencar S, Garnica M, Luiz RR, Nogueira CM, Borojevic R, Maiolino A, et al. Cryopreservation of peripheral blood stem cell: the influence of cell concentration on cellular and hematopoietic recovery. Transfusion. 2010;50:2402–12.
Akkök CA, Liseth K, Nesthus I, Løkeland T, Tefre K, Bruserud O, et al. Autologous peripheral blood progenitor cells cryopreserved with 5 and 10 percent dimethyl sulfoxide alone give comparable hematopoietic reconstitution after transplantation. Transfusion. 2008;48:877–83.
Mitrus I, Smagur A, Giebel S, Gliwinska J, Prokop M, Glowala-Kosinska M, et al. A faster reconstitution of hematopoiesis after autologous transplantation of hematopoietic cells cryopreserved in 7.5% dimethyl sulfoxide if compared to 10% dimethyl sulfoxide containing medium. Cryobiology. 2013;67:327–31.
Giebel S, Kruzel T, Czerw T, Sadus-Wojciechowska M, Najda J, Chmielowska E, et al. Intermediate-dose Ara-C plus G-CSF for stem cell mobilization in patients with lymphoid malignancies, including predicted poor mobilizers. Bone Marrow Transplant. 2013;48:915–21.
Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26.
Smagur A, Mitrus I, Giebel S, Sadus-Wojciechowska M, Najda J, Kruzel, et al. Impact of different dimethyl sulphoxide concentrations on cell recovery, viability and clonogenic potential of cryopreserved peripheral blood hematopoietic stem and progenitor cells. Vox Sang. 2013;104:240–7.
Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463–72.
Rosskopf K, Ragg SJ, Worel N, Grommé M, Preijers FW, Braakman E, et al. Quality controls of cryopreserved haematopoietic progenitor cells (peripheral blood, cord blood, bone marrow). Vox Sang. 2011;101:255–75.
Bakken AM, Bruserud O, Abrahamsen JF. No differences in colony formation of peripheral blood stem cells frozen with 5 or 10% dimethyl sulfoxide. J Hematother Stem Cell Res. 2003;12:351–8.
Lanza F, Campioni DC, Hellmann A, Milone G, Wahlin A, Walewski J, et al. Individual quality assessment of autografting by probability estimation for clinical endpoints: a prospective validation study from the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2013;19:1670–6.
Cox MA, Kastrup J, Hrubiško M. Historical perspectives and the future of adverse reactions associated with haemopoietic stem cells cryopreserved with dimethyl sulfoxide. Cell Tissue Bank. 2012;13:203–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mitrus, I., Smagur, A., Fidyk, W. et al. Reduction of DMSO concentration in cryopreservation mixture from 10% to 7.5% and 5% has no impact on engraftment after autologous peripheral blood stem cell transplantation: results of a prospective, randomized study. Bone Marrow Transplant 53, 274–280 (2018). https://doi.org/10.1038/s41409-017-0056-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-017-0056-6
This article is cited by
-
Application of RapidHIT™ ID for cell authentication by fast and convenient STR profiling
Genes & Genomics (2023)
-
Tandem autologous hematopoietic cell transplantation with sequential use of total marrow irradiation and high-dose melphalan in multiple myeloma
Bone Marrow Transplantation (2021)