Abstract
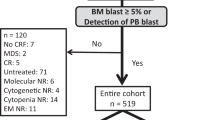
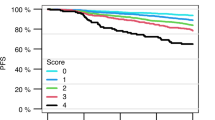
Predicting mobilization failure before it starts may enable patient-tailored strategies. Although consensus criteria for predicted PM (pPM) are available, their predictive performance has never been measured on real data. We retrospectively collected and analyzed 1318 mobilization procedures performed for MM and lymphoma patients in the plerixafor era. In our sample, 180/1318 (13.7%) were PM. The score resulting from published pPM criteria had sufficient performance for predicting PM, as measured by AUC (0.67, 95%CI: 0.63–0.72). We developed a new prediction model from multivariate analysis whose score (pPM-score) resulted in better AUC (0.80, 95%CI: 0.76–0.84, p < 0001). pPM-score included as risk factors: increasing age, diagnosis of NHL, positive bone marrow biopsy or cytopenias before mobilization, previous mobilization failure, priming strategy with G-CSF alone, or without upfront plerixafor. A simplified version of pPM-score was categorized using a cut-off to maximize positive likelihood ratio (15.7, 95%CI: 9.9–24.8); specificity was 98% (95%CI: 97–98.7%), sensitivity 31.7% (95%CI: 24.9–39%); positive predictive value in our sample was 71.3% (95%CI: 60–80.8%). Simplified pPM-score can “rule in” patients at very high risk for PM before starting mobilization, allowing changes in clinical management, such as choice of alternative priming strategies, to avoid highly likely mobilization failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transpl. 2016;51:786–92.
Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9.
Hubel K, Fresen MM, Apperley JF, Basak GW, Douglas KW, Gabriel IH, et al. European data on stem cell mobilization with plerixafor in non–Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma patients. A subgroup analysis of the European Consortium of stem cell mobilization. Bone Marrow Transpl. 2012;47:1046–50.
Perseghin P, Terruzzi E, Dassi M, Baldini V, Parma M, Coluccia P, et al. Management of poor peripheral blood stem cell mobilization: incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41:33–7.
Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transpl. 2008;14:1045–56.
Milone G, Martino M, Spadaro A, Leotta S, Di Marco A, Scalzulli P, et al. Plerixafor on demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest without increase in costs. Br J Haematol. 2013;164:113–23.
Farina L, Guidetti A, Spina F, Roncari L, Longoni P, Ravagnani F, et al. Plerixafor ‘on demand’: results of a strategy based on peripheral blood CD341 cells in lymphoma patients at first or subsequent mobilization with chemotherapy + G-CSF. Bone Marrow Transpl. 2014;9:453–5.
Sancho JM, Morgades M, Grifols JR, Juncà J, Guardia R, Vives S, et al. Predictive factors for poor peripheral blood stem cell mobilization and peak CD34(+) cell count to guide pre–emptive or immediate rescue mobilization. Cytotherapy. 2012;4:823–9.
Lanza F, Lemoli RM, Olivieri A, Laszlo D, Martino M, Specchia G, et al. Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 15 patients with multiple myeloma and lymphoma. Transfusion. 2013;54:31–339.
Sinha S, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Majority of patients receiving initial therapy with lenalidomide–based regimens can be successfully mobilized with appropriate mobilization strategies. Leukemia. 2012;26:1119–22.
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International Myeloma Working Group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high–dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009;23:1904–12.
Attolico I, Pavone V, Ostuni A, Rossini B, Musso M, Crescimanno A, et al. Plerixafor added to chemotherapy plus G–CSF is safe and allows adequate PBSC collection in predicted poor mobilizer patients with multiple myeloma or lymphoma. Biol Blood Marrow Transpl. 2012;18:241–9.
Efron B, Tibshirani RJ. An Introduction to the bootstrap. Boca Raton, FL. CRC press, 1994.
Olivieri A, Marchetti M, Lemoli R, Tarella C, Iacone A, Lanza F, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo Italiano Trapianto di Midollo Osseo. Bone Marrow Transpl. 2012;47:342–51.
Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. Risk–based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transpl. 2012;47:83–487.
Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transpl. 2011;6:64–69.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
DeLong ER, DeLong DM, Clarke–Pearson. DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Trajman A, Luiz RR. McNemar chi2 test revisited: comparing sensitivity and specificity of diagnostic examinations. Scand J Clin Lab Invest. 2008;68:77–80.
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin–Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65.
Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm for plerixafor use in autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transpl. 2013;19:87–93.
Horwitz ME, Chute JP, Gasparetto C, Long GD, McDonald C, Morris A, et al. Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration. Bone Marrow Transpl. 2012;47:1051–5.
Gambell P, Herbert K, Dickinson M, Stokes K, Bressel M, Wall D, et al. Peripheral blood CD34+cell enumeration as a predictor of apheresis yield: an analysis of over 1000 collections. Biol Blood Marrow Transpl. 2012;18:763–72.
Pierelli L, Perseghin P, Marchetti M, Accorsi P, Fanin R, Messina C, et al. Best practice for peripheral blood progenitor cell mobilization and collection in adults and children: results of a Società Italiana Di Emaferesi e Manipolazione Cellulare (SIDEM) and Gruppo Italiano Trapianto Midollo Osseo (GITMO) consensus process. Transfusion. 2012;52:893–905.
Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transpl. 2014;20:295–308.
Chow E, Rao KV, Wood WA, Covington D, Armistead PM, Coghill J, et al. Effectiveness of an algorithm-based approach to the utilization of plerixafor in patients undergoing chemotherapy-based stem cell mobilization. Biol Blood Marrow Transpl. 2014;20:1064–8.
Mohty M, Hübel K, Kröger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:865–72.
Whitaker R. Validation examples of the analytic hierarchy process and analytic network process. Math Comput Model. 2007;46:840–59.
Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–11.
Ferrante di Ruffano L, Hyde C, McCaffery KJ, Bossuyt PM, Deeks JJ. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ. 2012;344:e686.
Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet. 2005;365:1500–5.
Olivieri A, Saraceni F. Mobilization policy in multiple myeloma: minimum target or law of redundancy? Two different approaches by the two sides of the Atlantic Ocean. Bone Marrow Transpl. 2016;51:348–50.
Yuan S, Wang S. How do we mobilize and collect autologous peripheral blood stem cells? Transfusion. 2017;57:13–23.
Steyerberg EW, Harrell FE Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81.
Acknowledgements
The authors are very grateful to Giuseppe Ausoni, Paola Brambilla, Saveria Capria, Gloria Margiotta Casaluci, Michele Cimminiello, Annarita Conconi, Carmela Cuomo, Katia Codeluppi, Mario Delia, Roberta Distefano, Annalisa Di Marco, Luca Facchini, Salvatore Gattillo, Maria Gozzer, Svitlana Gumenyuk, Francesco Marchesi, Giovanna Meloni, Angela Melpignano, Luca Nassi, Domenico Pastore, Giuseppe Pietrantuono, Michele Pizzuti, Giovanni Quarta, Azzurra Anna Romeo, Federica Sorà, Andrea Spadaro and Stefania Trinca for their contribution to this study.
Author contributions
J.O. performed the statistical analysis and wrote the manuscript; A.O. contributed to study design, interpreted the results, contributed to manuscript writing, reviewed, and approved the manuscript; E.D.N. contributed to study design and to the statistical analysis; F.S. contributed to data collection and interpretation of the results, approved and edited the manuscript; I.A., M.C., M.P., P.P., P.E.P. contributed to data collection and interpretation of the results; P.C., L.F., G.G., L.N., S.S., N.P., M.M., T.M., M.P., F.Z., F.C., S.M., A.M., P.M., S.C., F.M., K.C., G.M., F.L., G.S., D.P., G.M. contributed to contributed to patient care and data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Olivieri, J., Attolico, I., Nuccorini, R. et al. Predicting failure of hematopoietic stem cell mobilization before it starts: the predicted poor mobilizer (pPM) score. Bone Marrow Transplant 53, 461–473 (2018). https://doi.org/10.1038/s41409-017-0051-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-017-0051-y
This article is cited by
-
A novel PEGylated form of granulocyte colony-stimulating factor, mecapegfilgrastim, for peripheral blood stem cell mobilization in patients with hematologic malignancies
BMC Cancer (2023)
-
Salvage treatment with plerixafor in poor mobilizing allogeneic stem cell donors: results of a prospective phase II-trial
Bone Marrow Transplantation (2021)
-
Collection and Processing of Mobilized Mouse Peripheral Blood at Lowered Oxygen Tension Yields Enhanced Numbers of Hematopoietic Stem Cells
Stem Cell Reviews and Reports (2020)
-
Pegfilgrastim improves the outcomes of mobilization and engraftment in autologous hematopoietic stem cell transplantation for the treatment of multiple myeloma
Annals of Hematology (2020)
-
The timing of plerixafor addition to G-Csf and chemotherapy affects immunological recovery after autologous stem cell transplant in multiple myeloma
Bone Marrow Transplantation (2020)