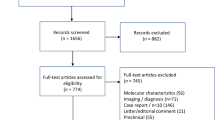
Abstract
When clonal plasma cells grow at anatomic sites distant from the bone marrow or grows contiguous from osseous lesions that break through the cortical bone, it is referred to as extramedullary multiple myeloma (EMD). EMD remains challenging from a therapeutic and biological perspective. The pathogenetic mechanisms are not completely understood and it is generally associated with high-risk cytogenetics which portends poor outcomes. There is a rising incidence of EMD in the era of novel agents, likely a reflection of longer OS, with no standard treatment approach. Patients benefit from aggressive chemotherapy-based approaches, but the OS and prognosis remains poor. RT has been used for palliative care. There is a need for large prospective trials for development of treatment approaches for treatment of EMD.
Similar content being viewed by others
Introduction
Multiple Myeloma (MM) is defined by the presence of ≥10% clonal bone marrow (BM) plasma cells (PC) associated with features of hypercalcemia, renal failure, anemia or lytic bone lesions or the presence of biomarkers such as ≥60% BMPC, involved to uninvolved FLC ratio ≥100, or the presence of ≥2 marrow lesions on MRI [1]. Despite high-dose chemotherapy with stem cell support (HDT) and novel therapeutic agents, prognosis remains poor. When a sub-clone of PCs is able to grow outside of marrow, it results in development of disease outside the marrow, termed as extramedullary multiple myeloma (EMD).
Classification
The term extramedullary can be confusing and as there is a lack of consensus regarding the classification, we put forward a convenient way to classify them in a manner that reflects the prognosis and the therapeutic approach (Table 1) [1,2,3,4,5,6]. EMD can be present either at initial diagnosis (primary EMD) or at relapse (secondary EMD) [3, 7].
The symptoms due to EMD are typically related to the site of lesions—a summary of literature regarding sites involved in EMD is provided in Table 2.
Epidemiology
Overall incidence of EMM is 13%:7% at diagnosis and 6–20% at relapse [8]. 85% of these are bone-associated and the median age for patients is higher as compared to patients with bone-independent EMD (71 vs 60.5 years) [2]. There has been an overall increase in the incidence of EMM from 6.5% in 2005 to 23.7% in 2014 [9]. Median time from diagnosis to occurrence of EMM has been observed to be 19–23 months [2, 8]. The results of total therapy protocol trials also reported that extra medullary involvement at presentation was more common among those with high-risk translocations t(14;16) and t(14;20) and was associated with poor overall survival (OS) [10].
Patients with osteolytic lesions and hypercalcemia are at a higher risk for developing EMD. Other significant risk factors include therapeutic history (>2 lines of treatment ± treatment duration >6 months) and allogenic SCT (auto-allo-SCT) [11, 12]. It is quite possible that the increasing frequency of EMD at relapse among patients with MM reflects the improved OS in general and that we are seeing a phase of the disease we did not reach before the advent of newer therapies.
Pathogenesis
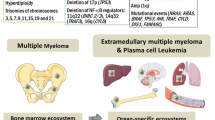
The interaction between myeloma cells and the BM microenvironment activates signaling cascades and mediates chemotaxis and adhesion of myeloma cells to BM (Fig. 1). The adhesion is augmented by binding of stromal-derived factor 1 a (SDF 1-A) to CXCR4 receptor and adhesion molecules like VLA-4, P-selectin, CD 56, and CD 44 [13]. Tumor dissemination occurs due to (i) low expression of chemokine receptors and adhesion molecules [4], (ii) underexpression of membrane-embedded CS81/CD 82 tetraspanins [14] and overexpression of tumor promoter heparanase enzyme, (iii) upregulation of CXCR4 by various growth factors and hypoxic conditions in tumor microenvironment [15] and acquisition of EM phenotype regulated by CXCR4 [15, 16]. A possible PCAT-1/Wnt β-catenin signaling axis has also been implicated in growth, OS, and migration of MM cells [17, 18]. Head and neck and liver have been reported as the most common location at diagnosis followed by pleural fluid at relapse [19]. It was hypothesized that specific tropism or homing of EMM clones makes them more prone to trafficking to these sites.
Recent studies have revealed that long non-coding RNA like MALAT1 and MEG-3 regulate gene expression at the transcriptional, post‐transcriptional, and epigenetic levels and are involved in tumor initiation, metastasis, and drug resistance [20]. MALAT1 located on chromosome 11 was observed to be markedly higher in EMD as compared to intramedullary MM cells [21]. It was observed that patients with a greater decrease in MALAT1 after initial treatment had a significantly prolonged progression‐free survival (PFS) duration, while patients with smaller MALAT1 changes after treatment had a significantly higher risk of early progression [21].
Immunophenotype
Studies have shown that EMDs have a higher proliferative index, lower p27 expression, and CCND-1 and p53 co-positivity [22]. BCl-2 and Bcl-xl are strongly positive, CD56 is downregulated and CD44 is upregulated [22, 23]. Immuno-phenotyping helps not only in identifying the cell but also in establishing the correct diagnosis.
Cytogenetic profile
Genetic aberrations in myeloma are usually identified using Fluorescence in-situ hybridization (FISH) and have an important prognostic value in MM. However, cytogenetic features of EMD are not well defined in literature. A few studies have reported association of high-risk cytogenetics like t(4;14), t(14;16), gain(1q21), and del(17p) in patients with EMD [2, 24, 25]. Studies have also identified del(17p13) and del(13q14) as markers for progression to EMD [2, 26] and del(13) as risk factor for EM relapse. Gain(1q) was associated with inferior outcome [27]. High risk cytogenetics was more frequent in patients with organ involvement (47%) vs EMM [28].
Clinical evaluation
Along with the routine myeloma workup, EMD requires a tumor biopsy/FNAC for immune-histochemistry (Table 3) and a BM biopsy to evaluate PC morphology and the degree of total PC infiltration [29]. Patients who develop EM spread during their disease course have significantly lower levels of serum M-protein and hemoglobin and significantly higher levels of lactate dehydrogenase (LDH) than those who present with EMD at diagnosis [8]. Using sensitive imaging techniques including MRI and PET/CT, EMD may be found in up to 30% of MM patients across the overall disease course.
Treatment
EMM
Radiotherapy (RT)
There is no consensus on use of RT in EMM except for SP. A few cases have reported the use of RT with good outcomes in EMD as outlined in Table 2.
Induction chemotherapy
With a rising incidence of EMD in the era of novel agents, it was hypothesized that newer drugs lead to drug resistant, inherently aggressive, and BM-independent clones [7]. However, there is no clinical evidence supporting the same [30]. Superior complete response rates in de-novo EMD patients have been reported with novel agents (thalidomide, lenalidomide and bortezomib-based regimens) vs conventional chemotherapy [31] (Table 4). In relapsed/refractory (r/R) patients with EMD, lymphoma-like polychemotherapy regimen such as PACE (cisplatin, doxorubicin, cyclophosphamide, and Etoposide), Dexa-BEAM, and HyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) followed by ASCT (ASCT) or auto-allo-SCT have been successful [32, 33]. Newer generation IMiDs such as pomalidomide have also been effective at the time of relapse [7]. Carfilzomib is also active but has inferior outcomes in bone-independent EMD compared to bone-related EMD [34]. One should consider the previous lines of therapy and the duration of response at relapse.
Extramedullary tumor masses in CNS most frequently arise from bone lesions in the cranial vault, skull base, nose, or paranasal sinuses, whereas primary dural (pachy-meningeal) involvement is rare. The OS with osteodural involvement (25 months) is three times more than leptomeningeal involvement (6 months) [35]. For CNS EMD, a combination of CNS directed treatment including RT and IT chemotherapy and systemic therapy including novel agents which can cross blood brain barrier (BBB) has shown activity [35, 36]. IMIDs are more likely to cross BBB than PIs and more prospective data is needed to determine ideal strategy [37].
There is paucity of data with use of Daratumumab in EMD. An updated pooled analysis of studies (GEN501 part 2 and SIRIUS) evaluating role of daratumumab in heavily pre-treated patients reported an overall response rate (ORR) in subset of patients with EM involvement was 16.7% (95% CI: 3.6–41.4) with improved OS in responders/minimal response/stable disease [38]. There are also several case reports with response to daratumumab in EMD.
Innovative approaches using adoptive cell therapies (chimeric antigen receptor T cells) have recently shown promising results in a limited number of relapsed patients with EMD [39]. In a meta-analysis on BCMA CAR-T cell therapy, the presence of EM disease at time of infusion was not associated with lower response rates showing a pooled response rate of 78% vs 82% overall [40]. The high response rates with anti-BCMA CAR-T therapy despite EM disease demonstrate the need for more focused subgroup analysis in upcoming CAR-T studies.
SCT
The preferred next step in patients who respond to induction therapy is transplant. However, the benefit of ASCT in patients with EMD appears to be more limited. The Spanish PETHEMA group observed a significantly shorter median OS (46.7 months vs NR) but no significant difference in 2-year PFS after ASCT with high-dose melphalan conditioning. The poor outcome after single ASCT can be attributed to high-risk cytogenetics which can be found in almost 40% patients with EMM. Single vs multiple sites of EMD as well as organ involved can also impact prognosis after ASCT [9]. Upfront tandem transplant has been shown to overcome poor outcomes in these patients compared to single ASCT [28]. Studies evaluating tandem transplantation suggest high-risk subgroups, including patients failing to achieve VGPR after single ASCT, International Staging System (ISS) stage II/III, and high-risk cytogenetics, may benefit most from tandem transplantation [41]. However, a EBMT registry study reported similar 3-year PFS and OS with both first-line tandem and single ASCT in patients with EMD [9, 42]. (Table 4).
Relapse after transplant
Patients with MM with EMD at diagnosis or during the disease course have a higher risk of EMD at relapse following HDT. The relapse rate is generally similar between bone-independent MM and bone-associated MM [24]. Various sites like bone, abdomen, and chest have been reported to be involved at the time of relapse [19, 24]. Although the mechanism is largely unclear, but worsening disease status at time of transplant may enhance the risk of EMM [43].
Gagelman et al. reported cumulative incidence of relapse in NDMM patients with EMD as 54% after single ASCT, 47% after tandem ASCT, and 30% after auto–allogeneic transplant [28]. Even though allo-SCT is associated with long-term disease-free OS, it is associated with high transplant-related mortality. A higher incidence of EM relapse (45–55%) has been observed with auto-allo-SCT with reduced intensity conditioning (RIC) [44]. A German study used auto-allo-SCT either as first line treatment or at the time of relapse as the escalation approach. They reported relapse in 49% of the patients with EMD present in one-third of the cohort. OS in EMD group was significantly inferior as compared to intramedullary relapse [45]. Allo-SCT takes advantage of a tumor cell-free graft along with the graft-versus-myeloma (GVM) effect targeting residual malignant plasma cells. Furthermore, allo-SCT allows for donor lymphocyte infusions as an additional intervention that has shown remarkable responses, clearly demonstrating the intensification of a GVM effect. Hence, in patients requiring rescue therapy, allo-RIC should be considered as a platform for additional therapeutic strategies after transplantation to take advantage of the GVM effect.
Prognosis
EM involvement is one of the indicators of poor prognosis in MM, with high mortality and an average OS time of 36 months [10, 35]. Factors causing worse progression-free OS and OS: (a) EMD, (b) EMD at relapse, (c) bone-independent EMD with MM, (d) multiple organ involvement, (e) CNS involvement, (f) No ASCT, (g) not achieved complete response post-SCT, (h) β2-microglobulin >5 mmol/L, (i) ISS II & III (j) acute GVHD [9, 31, 35, 44, 46]. On multivariate analysis, Shin et al. also reported platelet counts as predictive of poor PFS and bone marrow plasma cell percentage as predictive for poor OS after ASCT [24].
Cause of death
The EBMT report on EM multiple myeloma observed non-relapse mortality (NRM) at three years in 3% patients with bone associated EMD, and 7% in patients with EM organ involvement. The main causes of death were relapse or progression (86.3%), infection (7.1%), secondary malignancy or post-SCT lymphoproliferative disorder (3.6%), organ damage or failure (1.8%) and toxicity (0.4%) [9].
Future considerations
EMD presents a spectrum of disease presentations in MM with ill-defined boundaries. There is an urgent need for consensus on criteria defining EMD. The incidence of EMD is largely underestimated due to lack of prospective studies on large cohorts. New guidelines should be formulated which provide algorithms for treatment and follow-up of EMD using RT, chemotherapy, and surgery considering category, location, and tumor size. Large, randomized multi-center studies with long follow up are required to assess the efficacy and safety of available treatment options. Newer drugs like monoclonal antibodies, immunotherapy, and BCL-2 inhibitors are also worth exploring.
References
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Billecke L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol. 2013;161:87–94.
Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99:360–4.
Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–12.
Fernández de Larrea C, Kyle RA, Durie BGM, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–91.
Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286–91.
Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–8.
Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2009;21:325–30.
Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103:890–7.
Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–7.
Mangiacavalli S, Pompa A, Ferretti V, Klersy C, Cocito F, Varettoni M, et al. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann Hematol. 2017;96:73–80.
Chong G, Byrnes G, Szer J, Grigg A. Extramedullary relapse after allogeneic bone marrow transplantation for haematological malignancy. Bone Marrow Transplant. 2000;26:1011–5.
Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–17.
Tohami T, Drucker L, Shapiro H, Radnay J, Lishner M. Overexpression of tetraspanins affects multiple myeloma cell survival and invasive potential. FASEB J. 2007;21:691–9.
Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94.
Jung O, Trapp-Stamborski V, Purushothaman A, Jin H, Wang H, Sanderson RD, et al. Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis. 2016;5:e202.
Qiang YW, Chen Y, Brown N, Hu B, Epstein J, Barlogie B, et al. Characterization of Wnt/β‐catenin signalling in osteoclasts in multiple myeloma. Br J Haematol. 2010;148:726–38.
Shen X, Zhang Y, Wu X, Guo Y, Shi W, Qi J, et al. Upregulated lncRNA-PCAT1 is closely related to clinical diagnosis of multiple myeloma as a predictive biomarker in serum. Cancer Biomark. 2017;18:257–63.
Weinstock M, Aljawai Y, Morgan EA, Laubach J, Gannon M, Roccaro AM, et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Haematol. 2015;169:851–8.
Zhuang W, Ge X, Yang S, Huang M, Zhuang W, Chen P, et al. Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells. 2015;33:1985–97.
Handa H, Kuroda Y, Kimura K, Masuda Y, Hattori H, Alkebsi L, et al. Long non‐coding RNA MALAT 1 is an inducible stress response gene associated with extramedullary spread and poor prognosis of multiple myeloma. Br J Haematol. 2017;179:449–60.
Kremer M, Ott G, Nathrath M, Specht K, Stecker K, Alexiou C, et al. Primary extramedullary plasmacytoma and multiple myeloma: phenotypic differences revealed by immunohistochemical analysis. J Pathol. 2005;205:92–101.
Dahl IM, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br J Haematol. 2002;116:273–7.
Shin HJ, Kim K, Lee JW, Song MK, Lee JJ, Lee HS, et al. Comparison of outcomes after autologous stem cell transplantation between myeloma patients with skeletal and soft tissue plasmacytoma. Eur J Haematol. 2014;93:414–21.
Besse L, Sedlarikova L, Greslikova H, Kupska R, Almasi M, Penka M, et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur J Haematol. 2016;97:93–100.
Avivi I, Cohen YC, Suska A, Shragai T, Mikala G, Garderet L, et al. Hematogenous extramedullary relapse in multiple myeloma—a multicenter retrospective study in 127 patients. Am J Hematol. 2019;94:1132–40.
Shin H-J, Kim K, Lee JJ, Song MK, Lee EY, Park SH, et al. The t (11; 14)(q13; q32) translocation as a poor prognostic parameter for autologous stem cell transplantation in myeloma patients with extramedullary plasmacytoma. Clin Lymphoma Myeloma Leuk. 2015;15:227–35.
Gagelmann N, Eikema DJ, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the Chronic Malignancies Working Party of the European Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25:2134–42.
Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11:10.
Varga C, Xie W, Laubach J, Ghobrial IM, O'Donnell EK, Weinstock M, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide-bortezomib combinations. Br J Haematol. 2015;169:843–50.
Kumar L, Gogi R, Patel AK, Mookerjee A, Sahoo RK, Malik PS, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transplant. 2017;52:1473–5.
Lakshman A, Singh PP, Rajkumar SV, Dispenzieri A, Lacy MQ, Gertz MA, et al. Efficacy of VDT PACE‐like regimens in treatment of relapsed/refractory multiple myeloma. Am J Hematol. 2018;93:179–86.
Rasche L, Strifler S, Duell J, Rosenwald A, Buck A, Maeder U, et al. The lymphoma-like polychemotherapy regimen “Dexa-BEAM” in advanced and extramedullary multiple myeloma. Annals Hematol. 2014;93:1207–14.
Zhou X, Flüchter P, Nickel K, Meckel K, Messerschmidt J, Böckle D, et al. Carfilzomib based treatment strategies in the management of relapsed/refractory multiple myeloma with extramedullary disease. Cancers. 2020;12:1035.
Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118:1574–84.
Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971–6.
Egan PA, Elder PT, Deighan WI, O’Connor SJM, Alexander HD. Multiple myeloma with central nervous system relapse. Haematologica. 2020;105:1780–90.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37–44.
Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, et al. NY-ESO-1–specific TCR–engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–21.
Gagelmann N, Ayuk F, Atanackovic D, Kröger N. B cell maturation antigen‐specific chimeric antigen receptor T cells for relapsed or refractory multiple myeloma: A meta‐analysis. Eur J Haematol. 2020;104:318–27.
Cavo M, Goldschmidt H, Rosinol L, Pantani L, Zweegman S, Salwender HJ, et al. Double vs single autologous stem cell transplantation for newly diagnosed multiple myeloma: long-term follow-up (10-years) analysis of randomized phase 3 studies. Am Soc Hematology 2018.
Cavo M, Salwender H, Rosiñol L, Moreau P, Petrucci MT, Blau IW, et al. Double vs single autologous stem cell transplantation after bortezomib-based induction regimens for multiple myeloma: an integrated analysis of patient-level data from phase European III studies. American Society of Hematology Washington, DC; 2013.
Pérez-Simón JA, Sureda A, Fernández-Aviles F, Sampol A, Cabrera JR, Caballero D, et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20:542–5.
Yin X, Tang L, Fan F, Jiang Q, Sun C, Hu Y. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 2018;18:62.
Rasche L, Röllig C, Stuhler G, Danhof S, Mielke S, Grigoleit GU, et al. Allogeneic hematopoietic cell transplantation in multiple myeloma: focus on longitudinal assessment of donor chimerism, extramedullary disease, and high-risk cytogenetic features. Biol Blood Marrow Transplant. 2016;22:1988–96.
Beksac M, Cengiz Seval G, Kanellias N, Coriu D, Rosinol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica 2019;105:201–8.
Burkat CN, Van Buren JJ, Lucarelli MJ. Characteristics of orbital multiple myeloma: a case report and literature review. Surv Ophthalmol. 2009;54:697–704.
Tahiliani N, Kataria P, Patel A, Kendre P. Proptosis and hemiplegia as an initial manifestation of multiple myeloma. J Postgrad Med. 2018;64:243–6.
Norlia, A. Recurrent multiple myeloma presenting as a breast plasmacytoma. Med J Malaysia. 2010;65:227–8.
Khan AM, Azar I, Najjar S, Bevington T, Mehdi S. A case of aggressive multiple myeloma with extramedullary involvement of the female reproductive system, thyroid and breasts. Case Rep Hematol. 2019;2019:7348504.
Singh K, Kumar P, Pruthy R, Goyal G. Multiple myeloma presenting as thyroid plasmacytoma. Indian J Med Paediatr Oncol. 2017;38:552–4.
You WS, Bhuta S. Myeloma of laryngeal cartilage: literature review and case study. Ear Nose Throat J. 2019;100:145561319861379–NP119.
Cantone E, Di Lullo AM, Marano L, Guadagno E, Mansueto G, Capriglione P, et al. Strategy for the treatment and follow-up of sinonasal solitary extramedullary plasmacytoma: a case series. J Med Case Rep. 2017;11:219.
D'Aguillo C, Soni RS, Gordhan C, Liu JK, Baredes S, Eloy JA, editors. Sinonasal extramedullary plasmacytoma: a systematic review of 175 patients. in International forum of allergy & rhinology. 2014. Wiley Online Library.
Yanamandra U, Deo P, Sahu KK, Nampoothiri RV, Gupta N, Prabhakaran A, et al. Clinicopathological profile of myelomatous pleural effusion: single-center real-world experience and review of literature. Clin Lymphoma Myeloma Leuk. 2019;19:183–9. e1
Oshima K, Kanda Y, Nannya Y, Kaneko M, Hamaki T, Suguro M, et al. Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol. 2001;67:1–5.
Abelman W, Virchis A, Yong K. Extramedullary myeloma representing as a pericardial effusion with tamponade: two case reports and a further review of 19 cases in the literature. Leukemia Lymphoma. 2005;46:137–42.
Wang X, Xie H, Zhang L. Multiple myeloma with onset of pancreas involvement: a case report. Medicine. 2019;98:e16567.
Chim CS, Wong WM, Nicholls J, Chung LP, Liang R. Extramedullary sites of involvement in hematologic malignancies: case 3. Hemorrhagic gastric plasmacytoma as the primary presentation in multiple myeloma. J Clin Oncol. 2002;20:344–7.
Karp S, Shareef D. Ascites as a presenting feature of multiple myeloma. J R Soc Med. 1987;80:182–4.
Yamashita K, Horiuchi T, Hayashida A, Tachibana H, Toki D, Kondo T. Multiple myeloma with testicular involvement: a case report. Urol Case Rep. 2019;26:100971.
Jurczyszyn A, Olszewska-Szopa M, Hungria V, Crusoe E, Pika T, Delforge M, et al. Cutaneous involvement in multiple myeloma: a multi-institutional retrospective study of 53 patients. Leuk Lymphoma. 2016;57:2071–6.
Evans LA, Jevremovic D, Nandakumar B, Dispenzieri A, Buadi FK, Dingli D, et al. Utilizing multiparametric flow cytometry in the diagnosis of patients with primary plasma cell leukemia. Am J Hematol. n/a(n/a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bansal, R., Rakshit, S. & Kumar, S. Extramedullary disease in multiple myeloma. Blood Cancer J. 11, 161 (2021). https://doi.org/10.1038/s41408-021-00527-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-021-00527-y
This article is cited by
-
Teclistamab in relapsed refractory multiple myeloma: multi-institutional real-world study
Blood Cancer Journal (2024)
-
Dynamic monitoring of myeloma minimal residual disease with targeted mass spectrometry
Blood Cancer Journal (2023)
-
Response-Adapted Therapy for Newly Diagnosed Multiple Myeloma
Current Hematologic Malignancy Reports (2023)
-
Clinical significance of high-dose chemotherapy with autologous stem cell transplantation in the era of novel agents in patients older than 65 years with multiple myeloma
Annals of Hematology (2023)
-
Phase II trial of daratumumab with DCEP in relapsed/refractory multiple myeloma patients with extramedullary disease
Journal of Hematology & Oncology (2022)