Abstract
Pre-emptive DLI (pDLI) is an effective strategy in lowering the risk of relapse without significantly increasing the risk of graft-versus-host disease (GVHD) in the case of T cell lineage mixed chimerism (MC) post allogeneic transplant in hematological malignancies. Many patients, however, fail to receive timely pDLI and have dismal outcomes, which are not taken into consideration. We compared long-term outcomes of 106 patients having T cell MC after day 60 and undergoing allogeneic stem cell allograft for acute leukemia from an unrelated donor (UD), with 111 patients having complete chimerism (CC). Fifty-three (56%) patients received prophylactic pDLI. Thirty-six patients (67%) had a response (RR), 17 (33%) had no response (NR), and fifty-two (54%) did not receive any pDLI (ND). OS was better in MC group as compared to CC (54% vs 43%, p = 0.04), mainly due to reduction in NRM (14% vs 25%, p = 0.05), and all grade acute and chronic GVHD. Within the MC group, response to pDLI was the only significant factor predicting OS, DFS, and relapses with NR and ND having unfavorable outcomes as compared to RR (p = 0.001). T cell MC in patients undergoing UD allografts with alemtuzumab is no longer an adverse prognostic factor, as compared to patients having CC, after timely implementation of pDLI.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation from unrelated donors (UD) using peripheral stem cell grafts (PBSC) is effective in hematological malignancies but has been associated with relatively high rates of both acute and chronic graft-versus-host disease (GVHD) resulting in inferior survival as compared to matched donors (MSD) [1, 2].
T-cell depletion, especially alemtuzumab, reduces the risk of GVHD following allogeneic hematopoietic stem cell transplantation (HSCT) [3], but has been associated with an increased frequency of mixed T cell chimerism (MC) [4, 5]. Although stable MC (T cell subset), because of bilateral tolerance, has been associated with less acute GVHD [4,5,6], progressive loss of donor chimerism is associated with graft rejection [7,8,9] and disease relapse [6, 10,11,12]. The incidence of relapses and rejection further varies according to the intensities of the conditioning regimen [13]. In patients with persistent MC, donor lymphocyte infusion (pDLI) has been utilized to pre-emptively convert patients to full donor and prevent relapse [8, 14,15,16,17]. Majority of the studies demonstrating patients receiving pDLI and achieving outcomes similar to patients having complete chimerism (CC-mainly responders) were in pediatric settings, while using antithymocyte globulin [18, 19] and chimerism in CD34/33 fraction. In adults and alemtuzumab-based settings [20], the effectiveness of this strategy was demonstrated in achieving complete donor chimerism, optimal tolerance and outcomes [21, 22] and better efficacy as compared to therapeutic DLI [23], in patients receiving reduced-intensity conditioning regimens. However, realistically many patients with MC actually fail to receive immunotherapy, and they continue to have poor prognosis [22], which was not taken into consideration
We thus wanted to compare MC as a group (including patients unable to receive pDLI) to patients having CC and see if it still remains a prognostic factor after the early introduction of pDLI.
Methods and materials
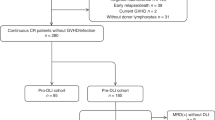
We retrospectively analyzed 224 patients who received first allo-HCT for acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), and acute lymphoblastic leukemia (ALL) transplanted from UD allograft (matched or mismatched) between the years 2007–2015. Out of 224 patients, 106 patients (47%) had T cell MC beyond day 60 of transplantation with complete lineage-specific chimerism (CD15/CD33/C19) and were compared to 111 patients having complete chimerism (CC) on day 60. Seven patients who could not be evaluated for day 60 chimerism were excluded from the analysis. All patients received in vivo T cell depletion with alemtuzumab. Patients having at least grade 2 GVHD prior to DLI were excluded from this strategy. The primary objective of this study was to analyze the long-term outcomes of patients with MC and whether pre-emptive DLI negates the adverse prognosis in terms of overall survival (OS). This study was reviewed and approved by the Institutional Review Boards of Kings College Hospital, and all patients were consented to as per the declaration of Helsinki.
Conditioning regimen and GVHD prophylaxis
The majority of patients (218/224, 97%) received peripheral blood stem cell (PBSC) grafts. Seventy-four 74/224 (33%)—[39/108 (37%) in the MC group, 52/115 (43%) in the CC group, p = 0.30] patients received 60 mg of alemtuzumab (given days -6 through -4), and 150/224 (67%), [69/108 (63%) patients MC group, 63/115 (57%) in the CC group] patients received 100 mg of alemtuzumab (given days-8 through -4). Alemtuzumab was given on days-3 to -1 for Bu/Cy and Cy/TBI regimens. Changes in dosing over time reflect systematic changes in institutional practice. Additional GVHD prophylaxis included a calcineurin inhibitor and methotrexate/mycophenolate mofetil (MMF) for ablative conditioning and a calcineurin inhibitor for reduced-intensity conditioning. Conditioning regimen consisted of fludarabine and high-dose busulfan (16 mg/kg orally or 12.8 mg/kg i.v.) (FB4) or cyclophosphamide (120 mg/kg) and total body irradiation (1200 cGy) (Cy/TBI) or busulfan (16 mg/kg orally, or 12.8 mg/kg i.v.) and cyclophosphamide (120 mg/kg) (Bu/Cy) for patients receiving ablative conditioning, and fludarabine and busulfan (6.4 mg/kg i.v.) (FB2), or fludarabine and melphalan (140 mg/m2) (FM) for patients receiving reduced-intensity conditioning (RIC). Pre-transplant disease risk was represented using the Disease Risk Index [24]. Acute GVHD was assessed using modified Glucksberg criteria, and chronic GVHD was classified as limited or extensive according to NIH criteria [25, 26].
Endpoints
OS was calculated from the time of transplant to the last follow-up date, Disease-free survival (DFS) was calculated as the time from transplantation and the earliest occurrence of any event relapse or death. Cytogenetic abnormality was classified according to the 2010 European Leukemia Net cytogenetic classification system [27]. The Chimerism was monitored using PCR amplification of a polymorphic STR region [28] every month for up to 90 days, and then every 3 months up to 2 years and then at 6-month intervals. Chimerism was separately analyzed for T cell subsets. The Indications for pDLI were falling T cell chimerism <50%. MC was defined as donor status less than 98% at day +60 (all data between day 50 to day 70). The first dose of pDLI was initiated at 5 × 105/kg for UD and 1 × 106/kg for siblings, and subsequent cycles were repeated at an interval of 6–8 weeks with escalating doses by half log each time, provided there was no evidence of active GVHD. The intention was to start pDLI by day 100. At least a 10% increase in chimerism, sustained at least for 2 months after the first pDLI was considered significant for a response (RR)
Statistical methods
Categorical variables were summarized as frequency counts and compared using chi-squared tests, and continuous variables were summarized as medians and compared using Mann–Whitney U-tests. OS and DFS were estimated by the Kaplan–Meier method and compared using the log-rank test. Cumulative incidences of relapse, non-relapse mortality (NRM), and GVHD were compared using the Finney and Grey model (25) and were considered as competing risks for each other (26). The univariate analysis included the following factors: conditioning intensity, type of donor, disease risk index, age, time to initiation of pDLI, chimerism prior to pDLI, and cell dose. Variables significant at the univariate level (p = or < 0.1), or variables which could affect final outcomes were then entered into a multivariate cox proportional hazard model and logistic regression. Statistical analysis was conducted using SPSS 24.0 software and R 3.4.3, and a p-value of 0.05 was used to indicate statistical significance, and all tests were two sided.
Results
Demographic profile
All patients treated between 2007 and 2015 were included in the analysis. Sixty-six patients (63%) patients having MC were age above 52 years. Within the patients having MC, 27 patients (22%) had mismatched 9/10 grafts, 83 (78%) patients had intermediate disease risk index, 42 (40%) patients had a myeloablative regimen, and 99 (93%) patients had myeloid disorder (48% MDS). The median follow-up period was 33 months (range, 0.6–150 months) for both the groups, (Table 1). The demographic distribution was similar among patients having CC.
Donor lymphocyte infusion, chimerism, and response
Fifty-three (56%) patients received pDLI. The median dose of pDLI was 1 × 106/kg (5 × 105/kg–1 × 107/kg) and the median time to pDLI was 5 months from transplantation (3.5–30 months). Patients received a median of 2 cycles of pDLI. The median CD3 at day 60 was 52%, with 37 (70%) of patients having CD3 chimerism <54%, (Supplementary Fig. 1). Out of 53 patients, 36 patients (67%) had a response (RR), and 17 (33%) patients had no response (NR). Fifty-two patients (54%) did not receive any DLI (ND). The main reason for patients unable to receive pDLI was either NRM (65% patients) or GVHD (unresolved grade 2 or higher grade GVHD, 28 %). Among 36 responders, the median time to best response was 7 months with the longest interval of the best response being around 100 months. Thirty-five patients among RR (98%) continued to maintain response beyond two months of starting pDLI and only one patient relapsed 5 months post-DLI and succumbed to progressive disease. None of the variables could significantly predict RR/NR.
Overall survival and disease-free survival, (OS, DFS) and relapses
5-year OS was worse in the CC group as compared to MC (43% vs 54%, p = 0.04). Two-year relapse and DFS between the groups were nearly similar (32% vs 38%, p = 0.99 and 38.5% vs 45%, p = 0.12, for CC and MC, respectively), (Fig. 1a–c). In multivariate analysis CC (ref MC, p = 0.02, HR: 1.53, CI: 1.0–2.2), older age, and mismatched transplant were independently predictive of the inferior OS, (Table 2). Among patients with MC, NR and ND patients did significantly worse as compared to RR in terms of DFS, OS, and relapse (p = 0.0001, HR = 5.45, 95% CI: 2.4–12, p = 0.001, HR-5.95, CI: 2.3–15, p = 0.0001, HR 9.45, CI: 2.8–30, respectively), (Fig. 2a–c, Table 3 and supplementary table 1).
Non-relapse mortality (2 years, NRM)
Two-year non-relapse treatment-related mortality (NRM) was significantly higher in the CC group (mainly related to GVHD) as compared to MC (25% vs 14%, p = 0.05). After MV analysis, CC (ref MC, p = 0.02, HR: 2.44, CI: 1.3–5.2), and older age were independent variables predicting higher NRM (Fig. 1d and Table 2). Within the MC group, infections (bacterial, viral, or fungal) and NRM were comparable between pDLI (RR/NR), and ND patients, (p = 0.37). There was 70% reactivation of CMV reactivation, without any significant CMV disease in patients who received pDLI, and there was no fungal death within 100 days of pDLI. DLI was well tolerated and with an incidence of only 6% overall NRM within the first 100 days among patients who had a response to pDLI. Among patients who received pDLI, 9 patients died of progression of the disease (8 patients having NR and 1 patient having RR), 1 patient died each of infection, severe acute GVHD, and secondary malignancy (all having RR). Among patients who did not receive pDLI primary cause of mortality was disease progression (85%). Only 3 patients receiving pDLI had long-term aplasia and they eventually relapsed (disease-related).
GVHD
All grade acute GVHD (I–IV) was significantly lower in the MC group (60% vs 38%, p < 0.001), however, acute severe GVHD (grade III and IV) was similar between patients having CC and MC (11% vs 13%, p = 0.83). Incidence of all grade cGVHD was significantly more in the CC group than MC (48% vs 37%, p = 0.05), although, severe extensive cGVHD was similar between the two (14% vs 11%, p = 0.32 for CC and MC, respectively), (Fig. 3a, b). The median time to develop chronic severe GVHD was 6 months (range, 3–64 months) in the MC group. Within the MC group, 14 (12%) patients had GVHD prior to initiation of pDLI (completely resolved at the time of initiation of pDLI, 5 experienced recurrence after pDLI). The two-year incidence of severe GVHD was 12% and grade 1–2 GVHD was 32%, respectively (among patients who received pDLI), and was comparable to ND (Table 3, Fig. 4). The median time to onset of GVHD in patients receiving pDLI was 4 months (3–11 months). Six patients (38%) needed some immunosuppression after pDLI due to GVHD. The presence/absence of GVHD did not alter outcomes (DFS/OS) for patients having MC or among patients who received pDLI (supplementary table 1). However, patients achieving RR without GVHD had significantly better outcomes than patients with MC having NR or RR with GVHD [22].
Discussion
T-cell depletion in UD grafts, especially alemtuzumab is associated with an increased frequency of mixed chimerism (MC) and relapses [4, 7, 11]. Pre-emptive DLI has been an effective strategy to prevent impending relapses without experiencing adverse graft vs host disease or infection in children and adults [15] However, studies have focused on comparing pDLI with patients having complete chimerism, not taking into account patients who fail to receive timely pDLI [15, 17] We retrospectively analyzed a large single-center dataset, of patients having mixed T cell chimerism (day 60) post allogeneic stem cell transplant in alemtuzumab-based setting, and compared their outcomes to patients having CC.
Overall, our results showed lower NRM associated with the MC group as compared to CC patients (mainly due to a reduction in overall GVHD). Despite relatively older patients (median age almost 60 years) and poor-disease risk index (61% high and intermediate risk), the non-relapse mortality was low (6% and 14% at 100 days and 1 year, respectively) among patients who received pDLI, and it was also associated with low rates of hospitalization as well as infections. Although CMV reactivation was commonly encountered, there were no cases of CMV disease, and no fungal infections or related deaths were seen in the first 100 days.
There was a significantly low incidence of all grades acute and chronic GVHD among patients who had MC as compared to patients having CC, though severe grade GVHD was comparable. The incidence of severe GVHD in patients receiving pDLI was comparable with that reported by Solomon et al. (12%) [21], wherein 36 patients, receiving alemtuzumab-based T cell depletion, received pDLI without withdrawal of immune suppression. It was lower than that reported by Bar et al. (28%) [29], where 35 patients prospectively received pDLI with pentostatin. The presence or absence of GVHD did not affect overall outcomes, similar to other groups. Although, severe GVHD was comparable between the two groups—probably the delay in onset of severe GVHD within the MC group might make it more amenable to treatment response and less aggressive as compared to early onset severe GVHD
OS was better in the MC group as compared to CC, especially among patients who received pDLI, and responded to the same (mainly due to reduction in NRM attributed to early GVHD within 100 days in the CC group). However, outcomes among patients within the MC group having ND and NR were particularly dismal. OS, DFS, and relapse, in our study, rates were significantly better for patients with MC as compared to other historical groups wherein pDLI was not implemented [4, 6, 12]. Within the MC group, the OS, DFS, and relapse rates of patients receiving pDLI or ND, in our cohort, was similar to that shown by Retrigger et al., (DFS: 66% for MC receiving pDLI, as compared to nearly 0% for patients not receiving DLI, p = 0.0009—among pediatric population using CD34/33 as chimerism fraction) [15], the study by Bar et al. (DFS: 45% for RR group vs 20% for NR) [21], and Solomon et al. (OS: 48% among 36 patients receiving pDLI) [29]. Also, we believe, that as outcomes in NR continues to remain very dismal, every attempt should be made in implementing additional pre-emptive novel agents (azacytidine/pentostatin) in the NR group. As some patients relapsing with leukemic blasts developed aGVHD despite the detection of MC or were critically ill, this recommended pre-emptive immunotherapy approach could not be offered to all MC patients. This is a drawback in most of the studies, and it would be worthwhile exploring other early immune-modulatory drugs (azacytidine) to further improve outcomes among patients having MC.
In univariate and multivariate analysis, the only factor predicting outcomes in patients with MC was the implementation of pDLI and the associated response, with the best outcomes among patients having a favorable response. The low CD3 percentage at day 60 of transplantation was not associated with response or overall outcomes, in contradiction to another study [16], wherein patients with high-risk disease had received only non-myeloablative conditioning. In a study by Bar et al. [29], where they used reduced-intensity conditioning along with pDLI with pentostatin, no such relation was found between CD3 and response. There was also no correlation in our study between the interval to initiation of pDLI and response or overall outcomes. Solomon et al. [21], in their study, showed a distinct improvement in outcomes in patients receiving pDLI within the first 100 days of transplantation. However, the subgroups were very small to have any definitive conclusions.
The retrospective nature of the study and a small subgroup of patients receiving pDLI (53 patients) among patients with MC, remains a potential drawback of this study. Furthermore, many patients in the MC group could not receive DLI due to the reasons mentioned above potentially biasing the results. Also, seven patients died prior to day 60 evaluation and were excluded from analysis, and although the number is very small it could still lead to a potential selection bias.
In conclusion, pre-emptive DLI (patients with T cell mixed chimerism after day 60) seems to be a successful and well-tolerated strategy in both the RIC and myeloablative setting to overcome adverse prognosis associated with MC, in adult patients with acute leukemia and undergoing unrelated donor transplant with alemtuzumab induced T cell depletion. Response to pDLI correlates strongly with final outcomes.
References
Weisdorf DJ, Nelson G, Lee SJ, Haagenson M, Spellman S, Antin JH, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009;15:1475–8.
Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
Mead AJ, Thomson KJ, Morris EC, Mohamedbhai S, Denovan S, Orti G, et al. HLA-mismatched unrelated donors are a viable alternate graft source for allogeneic transplantation following alemtuzumab-based reduced-intensity conditioning. Blood. 2010;115:5147–53.
Mattsson J, Uzunel M, Remberger M, Ringden O. T cell mixed chimerism is significantly correlated to a decreased risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2001;71:433–9.
van Besien K, Dew A, Lin S, Joseph L, Godley LA, Larson RA, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–17.
Mohty M, Avinens O, Faucher C, Viens P, Blaise D, Eliaou JF. Predictive factors and impact of full donor T-cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Haematologica. 2007;92:1004–6.
Keil F, Prinz E, Moser K, Mannhalter C, Kalhs P, Worel N, et al. Rapid establishment of long-term culture-initiating cells of donor origin after nonmyeloablative allogeneic hematopoietic stem-cell transplantation, and significant prognostic impact of donor T-cell chimerism on stable engraftment and progression-free survival. Transplantation. 2003;76:230–6.
Bethge WA, Hegenbart U, Stuart MJ, Storer BE, Maris MB, Flowers ME, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103:790–5.
Saito AM, Kami M, Mori S, Kanda Y, Suzuki R, Mineishi S, et al. Prospective phase II trial to evaluate the complications and kinetics of chimerism induction following allogeneic hematopoietic stem cell transplantation with fludarabine and busulfan. Am J Hematol. 2007;82:873–80.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003.
Shaw BE, Byrne JL, Das-Gupta E, Carter GI, Russell NH. The impact of chimerism patterns and predonor leukocyte infusion lymphopenia on survival following T cell-depleted reduced intensity conditioned transplants. Biol Blood Marrow Transplant. 2007;13:550–9.
Barrios M, Jiménez-Velasco A, Román-Gómez J, Madrigal ME, Castillejo JA, Torres A, et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica. 2003;88:801–10.
Mackinnon S, Barnett L, Heller G, O’Reilly RJ. Minimal residual disease is more common in patients who have mixed T-cell chimerism after bone marrow transplantation for chronic myelogenous leukemia. Blood. 1994;83:3409–16.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Kremens B, Dilloo D, et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant. 2004;33:815–21.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–8.
Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–9.
Horn B, Petrovic A, Wahlstrom J, Dvorak CC, Kong D, Hwang J, et al. Chimerism-based pre-emptive immunotherapy with fast withdrawal of immunosuppression and donor lymphocyte infusions after allogeneic stem cell transplantation for pediatric hematologic malignancies. Biol Blood Marrow Transplant. 2015;21:729–37.
Bader P, Beck J, Schlegel PG, Handgretinger R, Niethammer D, Klingebiel T. Additional immunotherapy on the basis of increasing mixed hematopoietic chimerism after allogeneic BMT in children with acute leukemia: is there an option to prevent relapse? Bone Marrow Transplant. 1997;20:79–81.
Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P, et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia. 1999;13:2079–86.
Mohamedbhai SG, Edwards N, Morris EC, Mackinnon S, Thomson KJ, Peggs KS. Predominant or complete recipient T-cell chimerism following alemtuzumab-based allogeneic transplantation is reversed by donor lymphocytes and not associated with graft failure. Br J Haematol. 2012;156:516–22.
Solomon SR, Sizemore CA, Zhang X, Brown S, Holland HK, Morris LE, et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: a prospective phase II trial. Bone Marrow Transplant. 2014;49:616–21.
Feliu J, Potter V, Grimaldi F, Clay J, Floro L, Saha C, et al. Full donor chimerism without graft-versus-host disease: the key factor for maximum benefit of pre-emptive donor lymphocyte infusions (pDLI). Bone Marrow Transplant. 2019;55:562–569.
Krishnamurthy P, Potter VT, Barber LD, Kulasekararaj AG, Lim ZY, Pearce RM, et al. Outcome of donor lymphocyte infusion after T cell-depleted allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2013;19:562–8.
Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13.
Martino R, Romero P, Subirá M, Bellido M, Altés A, Sureda A, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24:283–7.
Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol Blood Marrow Transplant. 2015;21:1343–59.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Kreyenberg H, Holle W, Mohrle S, Niethammer D, Bader P. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: the Tuebingen experience. Leukemia. 2003;17:237–40.
Bar M, Flowers M, Storer BE, Chauncey TR, Pulsipher MA, Thakar MS, et al. Reversal of low donor chimerism after hematopoietic cell transplantation using pentostatin and donor lymphocyte infusion: a prospective phase II multicenter trial. Biol Blood Marrow Transplant. 2018;24:308–13.
Author information
Authors and Affiliations
Contributions
V.S.: collected data, conceptualized idea and proposal, analyzed data, prepared manuscript, designed study. V.P., H.D., S.G., A.K., P.K., V.M., F.D., T.P., G.M.: treated patients and reviewed the manuscript. D.M., K.R.: designed study and reviewed/analyzed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheth, V., Potter, V., de Lavallade, H. et al. Mixed T cell lineage chimerism in acute leukemia/MDS using pre-emptive donor lymphocyte infusion strategy—Is it prognostic?—a single-center retrospective study. Blood Cancer J. 11, 128 (2021). https://doi.org/10.1038/s41408-021-00519-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-021-00519-y
This article is cited by
-
Prophylactic versus Preemptive modified donor lymphocyte infusion for high-risk acute leukemia after allogeneic hematopoietic stem cell transplantation: a multicenter retrospective study
Bone Marrow Transplantation (2024)
-
Pre-emptive and prophylactic donor lymphocyte infusion following allogeneic stem cell transplantation
International Journal of Hematology (2023)