Abstract
Low-dose thalidomide and prednisone alone or combined are effective therapies in some persons with primary myelofibrosis (PMF) and anemia with or with RBC transfusion dependence. Danazol is also effective in some persons with PMF and anemia. Responses to these drugs are typically incomplete and not sustained. It is unclear whether adding danazol to thalidomide and prednisone would improve efficacy. We retrospectively compared the outcomes of 88 subjects with PMF and anemia receiving thalidomide and prednisone without (n = 46) or with danazol (n = 42). The primary end point was anemia response, which was 71% (95% confidence interval (CI), 57, 85%) in subjects receiving thalidomide/prednisone/danazol compared with 46% (32, 60%; P = 0.014) in those receiving thalidomide/prednisone. Response rates in subjects who were RBC transfusion dependent was also higher in the danazol cohort (61% (38, 84%)) vs. 25% (6, 44%); P = 0.024). Time to response was rapid (median, 2 months (range, 1–11 months)) and similar between the cohorts. Response duration was longer in the thalidomide/prednisone/danazol cohort (HR 2.18 (1.18–5.42); P = 0.019). Adverse effects were mild and similar between the cohorts. In conclusion, thalidomide/prednisone/danazol seems superior to thalidomide/prednisone in persons with PMF and anemia. Our conclusion requires confirmation in a randomized trial.
Similar content being viewed by others
Introduction
About one-third of persons with primary myelofibrosis (PMF) have anemia at diagnosis and it develops in most others as the disease evolves1,2. Anemia and red blood cell (RBC) transfusion dependence are independent adverse prognostic variables for survival1,2,3. Erythropoiesis-stimulating agents (ESAs), androgenic steroids, thalidomide, lenalidomide, splenectomy, and prednisone are only modest activity in reversing anemia and ruxolitinib, pacritinib and fedratinib typically worsen anemia4,5,6,7. New effective therapies are needed.
Thalidomide is active in PMF because of its anti-angiogenic, cytokine regulatory, and immune-modulating properties8,9. Thalidomide, 100–400 mg/day, is reported to improve anemia in 20–60% of subjects10,11,12. However, these doses are associated with substantial toxicity and are poorly tolerated10,11. The combination of low-dose thalidomide, 50 mg/day, and prednisone is better tolerated and results in slightly higher responses than thalidomide alone13.
Androgenic steroids reverse anemia by stimulating erythropoietin, increasing iron use and reversing telomere loss14,15. Danazol, a synthetic androgen with reduced masculinizing activity, is reported to reverse anemia and thrombocytopenia in persons with PMF16,17. Combining therapies with different mechanisms of action might be even more effective in reversing anemia. Herein, we report efficacy, safety, and long-time outcome of therapy with low-dose thalidomide and prednisone with or without danazol in subjects with PMF and anemia with or without RBC transfusion dependence.
Subjects and methods
Subjects
This study was approved by the Ethical Committee of Institute of Hematology, CAMS and PUMC according to guidelines of the Declaration of Helsinki. From March 2006 to September 2016, 88 consecutive subjects and anemia with or without RBC transfusion dependence were enrolled. Eligibility criteria were: (1) PMF according to the WHO 2016 criteria18; (2) age ≥ 18 years; (3) hemoglobin concentration <100 g/L or RBC transfusion dependence19; (4) no exposure to ESAs, androgens, thalidomide, lenalidomide, or corticosteroids <12 weeks pre-enrollment; (5) creatinine ≤2 mg/dL, direct bilirubin <2 times upper limit of normal (ULN) and alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ≤3 ULN. Subjects receiving ruxolitinib during the past several years were excluded. Subjects had a pre-therapy physical examination, baseline laboratory assessment of serum chemistries and blood hematologic parameters, bone marrow aspirate and biopsy, and cytogenetic analyses. Prognostic cohort was assigned using the Dynamic International Prognostic Scoring System (DIPSS)2 for all subjects and DIPSS-plus3 for those with cytogenetics data. Bone marrow fibrosis was graded using European consensus guidelines20.
Therapy
Subjects received thalidomide, 50 mg p.o. at bed time continuously. Prednisone, 0.5 mg/kg/day, was given for 1 month, 0.25 mg/kg/day, for the next month, 0.125 mg/kg/day for the third month and tapered thereafter. Danazol, 600 mg/day p.o. was given continuously. Patients with neutrophil count <1.0 × 10E + 9/L and/or platelet count <80 × 10E + 9/L were assigned to thalidomide/prednisone/danazol therapy, the others were assigned to thalidomide/prednisone therapy. Laboratory studies were performed weekly for 12 weeks. Responders continued on their assigned therapy, whereas others stopped. Packed RBCs were transfused for a hemoglobin concentration <60 g/L or symptoms of anemia.
Evaluation of response and adverse events (AEs)
The primary study outcome was anemia response. In subjects with splenomegaly or thrombocytopenia, spleen and platelet responses were also analyzed. Anemia and spleen responses were assessed according to the revised International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) consensus criteria21. Thrombocytopenia response was defined as a platelet increase >50 × 10E + 9/L in subjects with baseline platelets <100 × 10E + 9/L. Toxicity was assessed by the National Cancer Institute Common Toxicity Criteria for Adverse Events, Version 422.
Statistical analyses
Follow-up was to death or 10 June 2017. Quantitative data are expressed as median and range and qualitative data as percent. Baseline variables were compared between cohorts using χ2 test for categorical variables and Mann–Whitney U-test for continuous variables. Clinical responses were compared between cohorts with χ2 test. Logistic regression was used to assess relationships between baseline variables and outcomes. Variables significant in univariate analyses were included in the multivariate analysis. Response duration was defined as the interval from anemia response to loss of response, change of therapy, or death. Response duration was calculated using the Kaplan–Meier method and compared by the log-rank test. P-values are two-sided and statistical significance defined as P < 0.05. Statistical analyses were performed using the IBM SPSS 22.0 package (SPSS, Chicago, IL, USA).
Results
Subject- and disease-related variables
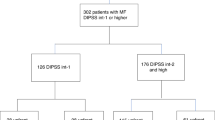
Eighty-eight subjects with PMF and anemia were evaluated. Forty-six received thalidomide and prednisone and 42 received thalidomide, prednisone, and danazol. Baseline variables were similar (Table 1). Median age was 53 years (range, 21–77 years). Forty-six (52%) were male. Thirty-eight subjects (43%) were RBC transfusion dependent19 and 49 (56%) had platelets <100 × 10E + 9/L. Median hemoglobin concentration and median white blood cell (WBC) and platelet levels were 71 g/L, 3.49 × 10E + 9/L and 84 × 10E + 9/L, respectively. Spleens were palpable a median of 4 cm (range, 0–24 cm) below the left costal margin (LCM). Fourteen of 54 subjects (26%) with evaluable cytogenetics had unfavorable karyotypes according to DIPSS-plus3. Thirty-one of 70 subjects tested had JAK2V617F. Forty-five subjects (51%) were intermediate-1, 38 (43%) were intermediate-2 and 5 (6%), high-risk according to the DIPSS2.
Thirty-one subjects received prior therapy(ies) including 9, thalidomide; 20, androgenic steroids; 5, recombinant human erythropoietin; 3, corticosteroids; 4, hydroxyurea; 3, interferon; and 1, melphalan. Median interval from diagnosis to study entry was 0 month (range, 0–62 months). Althought on-study, nine subjects received hydroxycarbamide (hydroxyurea) alone (N = 7) or with interferon (N = 2) and two received melphalan.
Responses and outcome
The anemia response rate for all subjects was 58% (95% confidence interval (CI) 48, 68%). Subjects receiving thalidomide/prednisone/danazol had a significantly higher response rate compared with those receiving thalidomide/prednisone (71% (57, 85%) vs. 46% (32, 60%); P = 0.014). Response rates in subjects who were RBC transfusion dependent were also higher in the danazol cohort (61% (38, 84%) vs. 25% (6, 44%); P = 0.024).
There is no significant correlations between anemia response rate and JAK2V17F mutation state (P = 0.238). Sixty-eight percent (52, 84%) of JAK2V617F subjects responded compared with 54% (38, 70%) JAK2 wild-type subjects (P = 0.238). In subgroup analysis, 63% (39, 87%) of JAK2V617F subjects responded compared with 42% (20, 64%) JAK2 wild-type subjects among patients receiving thalidomide/prednisone (P = 0.229), 73% (51, 95%) of JAK2V617F subjects responded compared with 65% (44, 86%) JAK2 wild-type subjects among patients receiving thalidomide/prednisone/danazol (P = 0.875).
In multivariate analyses, only thalidomide/prednisone/danazol therapy (odds ratio (OR) = 3.39 (1.29, 8.89); P = 0.013) and not being RBC transfusion dependent (OR = 2.90 (1.11, 7.61); P = 0.03) were significantly associated with response (Table 2). Responses occurred rapidly: median time to response was 2 months (range, 1–11 months) and did not differ between the cohorts. Interval to response varied: 61% of responders did so by 3 months, 94% by 6 months and only 6% after 6 months.
The minimum duration of treatment was 3 months, with a median of 25 months (range 3–117+ months). Subjects receiving thalidomide/prednisone/danazol had significantly longer response durations than those receiving thalidomide/prednisone (hazard ratio (HR) 2.18, 95%CI (1.18–5.42), P = 0.019; Fig. 1). Median response duration was 27 months (95% CI, 15–39 months) overall, 30 months (10–49 months) in the thalidomide/prednisone/danazol cohort compared with 11 months (0–30 months) in the thalidomide/prednisone cohort.
Twenty-two of 49 subjects (45% (31, 59%)) with baseline platelets <100 × 10E + 9/L had an increase of >50 × 10E + 9 L including 58% (39, 77%) of subjects receiving thalidomide/prednisone/danazol vs. 30% (11, 49%; P = 0.06) of subject in the thalidomide/prednisone cohort. Median time to platelet response was 3 months (range, <1–9 months) and was similar between the cohorts as was response duration (21 months (95% CI, 7–35 months)). There was a spleen response in 16 of 41 evaluable subjects (39% (24, 54%)) with similar response rates between the cohorts.
Adverse events
AEs were dose dependent and reversible with similar incidences (save ALT/AST increases) and severities in the cohorts. No subject discontinued therapy because of drug-related AEs. Leukocytosis and thrombocytosis occurred in 19% (11, 27%) and 24% (15, 33%) of subjects. There was no thrombo-embolic event. The most frequent non-hematologic AE was increased ALT/AST in 19% (8, 31%) of subjects receiving thalidomide/prednisone/danazol compared with 4% (3, 22%; P = 0.07) of subjects receiving thalidomide/prednisone. Other non-hematologic AEs were less frequent and did not differ significantly between the cohorts (Table 3) including increased bilirubin in four, constipation in six, hyperglycemia in six; rash in five; edema in six, neurological symptoms in seven; abdominal distention in four; hypertension in three, somnolence in two, and creatinine elevation in two. There was no case of prostate cancer or hepatic adenoma.
Discussion
Efficacy of low-dose thalidomide/prednisone in persons with PMF and anemia was studied in relatively small series of subjects with variable response criteria and response rates13,23,24. We confirmed the efficacy of thalidomide/prednisone using the current IWG-MRT criteria21. Importantly, adding danazol significantly increased response. In another recent study, danazol alone was reported to have a response rate of 30% (17, 43%)17. These data suggest therapy with thalidomide/prednisone/danazol is better than any component therapy.
Previous studies reported lower serum EPO levels, smaller spleen size, and lower RBC transfusion frequency were associated with higher anemia response rates17,25,26. We found such an association only for RBC transfusion dependence, and independence. We also found an advantage for combined therapy in subjects who were RBC transfusion dependent. Anemia responses occurred quickly and similarly in the two groups. Median time to response was shorter in our study than a previous study of danazol17 suggesting thalidomide/prednisone may have accelerated danazol responses.
Our data suggest adding danazol to thalidomide/prednisone improves response rates, prolongs response duration in persons with PMF and anemia with and without RBC transfusion dependence. The major limitation of our study is that it was a retrospective analysis and not randomized, and that although the cohorts were similar for known predictive variables we cannot be certain they were comparable for unknown predictive variables. As such, our conclusions require confirmation in a randomized trial.
References
Cervantes, F. et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood 113, 2895–2901 (2009).
Passamonti, F. et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115, 1703–1708 (2010).
Gangat, N. et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 29, 392–397 (2011).
Cervantes, F. How I treat myelofibrosis. Blood 124, 2635–2642 (2014).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807 (2012).
Komrokji, R. S. et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood 125, 2649–2655 (2015).
Pardanani, A. et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 1, 643–651 (2015).
D’Amato, R. J., Loughnan, M. S., Flynn, E. & Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 91, 4082–4085 (1994).
Thomas, D. A. et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer Am. Cancer Soc. 106, 1974–1984 (2006).
Elliott, M. A. et al. Thalidomide treatment in myelofibrosis with myeloid metaplasia. Br. J. Haematol. 117, 288–296 (2002).
Strupp, C. et al. Thalidomide for the treatment of idiopathic myelofibrosis. Eur. J. Haematol. 72, 52–57 (2004).
Barosi, G. et al. Safety and efficacy of thalidomide in patients with myelofibrosis with myeloid metaplasia. Br. J. Haematol. 114, 78–83 (2001).
Mesa, R. A. et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood 101, 2534–2541 (2003).
Bachman, E. et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A. Biol. Sci. Med. Sci. 69, 725–735 (2014).
Townsley, D. M. et al. Danazol treatment for telomere diseases. N. Engl. J. Med. 374, 1922–1931 (2016).
Cervantes, F., Alvarez-Larran, A., Domingo, A., Arellano-Rodrigo, E. & Montserrat, E. Efficacy and tolerability of danazol as a treatment for the anemia of myelofibrosis with myeloid metaplasia: long-term results in 30 patients. Br. J. Haematol. 129, 771–775 (2005).
Cervantes, F. et al. Danazol therapy for the anemia of myelofibrosis: assessment of efficacy with current criteria of response and long-term results. Ann. Hematol. 94, 1791–1796 (2015).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Gale, R. P. et al. What are RBC-transfusion-dependence and -independence? [Letter]. Leuk. Res. 35, 8–11 (2011).
Thiele, J. et al. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90, 1128–1132 (2005).
Tefferi, A. et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 122, 1395–1398 (2013).
US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute 2009, 4. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed September 6, 2015.
Mesa, R. A., Elliott, M. A., Schroeder, G. & Tefferi, A. Durable responses to thalidomide-based drug therapy for myelofibrosis with myeloid metaplasia. Mayo Clin. Proc. 79, 883–889 (2004).
Thapaliya, P. et al. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens [letter]. Am. J. Hematol. 86, 96–98 (2011).
Cervantes, F. et al. Erythropoietin treatment of the anemia of myelofibrosis with myeloid metaplasia: results in 20 patients and review of the literature. Br. J. Haematol. 127, 399–403 (2004).
Tsiara, S. N., Chaidos, A., Bourantas, L. K., Kapsali, H. D. & Bourantas, K. L. Recombinant human erythropoietin for the treatment of anemia in patients with chronic idiopathic myelofibrosis. Acta Haematol. 117, 156–161 (2007).
Acknowledgements
This study was supported in part by National Natural Science Funds (no. 81530008, no. 81370611, no. 81270585, and no. 81470297), Program for Peking Union Scholars and Innovative Research Team and PUMC Youth Fund and Fundamental Research Funds for the Central Universities (no. 3332016089). R.P.G. acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.P.G. is a part-time employee of Celgene Corp.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, X., Xu, Z., Li, B. et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer Journal 8, 9 (2018). https://doi.org/10.1038/s41408-017-0029-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-017-0029-4
This article is cited by
-
Management of Myelofibrosis-Associated Anemia: Focus on Standard Agents and Novel Therapeutics in Phase 3 Clinical Trials
Current Hematologic Malignancy Reports (2021)
-
Ruxolitinib-based combinations in the treatment of myelofibrosis: worth looking forward to
Annals of Hematology (2020)
-
Anemia in myelofibrosis—prevalence, the U2AF1 connection, new treatments
Blood Cancer Journal (2017)