Abstract
Aim
To review evidence on oral health practices, beliefs/views and experiences of community-dwelling older adults living with dementia, including their carers.
Materials and methods
A search of key terms across six databases including Pubmed, Web of Science and OVID (Embase, MEDLINE [R] and PsycINFO) and Google Scholar was conducted, supplemented by reference screening. The Mixed Methods Appraisal Tool (MMAT) 2018 was used to assess the methodological quality.
Results
Eighteen studies reported across 19 papers were included in the review. Papers largely focused on normative needs (n = 13), whilst also reporting oral health-related experiences (n = 2), practices (n = 7), and beliefs/views (n = 9), of community dwellers with dementia. Generally, people living with dementia presented with poor oral and dental health, the exception being one study where dental care was integrated with memory clinic services. Maintenance of oral health focused only on toothbrushing. Overall, people living with dementia have reduced capacity for self-performed oral hygiene and high reliance on caregivers. There was a paucity of evidence on their perceptions of oral health and quality of life, the findings of which were equivocal, with weak evidence suggesting possible difficulty in identifying and communicating their needs. Experiences of accessing dental care, when explored, appear to be system dependent.
Conclusion
There was limited research evidence on oral health-related practices, beliefs/views and experiences of people with dementia. Recommendations for future research are presented.
Similar content being viewed by others
Introduction
Dementia is one of the biggest health and social care challenges facing the world today. Globally, an estimated 50 million people are currently diagnosed with dementia, with a predicted threefold increase by the year 2050.1
As a neurodegenerative disorder, dementia is characterised by a chronic decline in cognitive and motor functions, which increases in risk with age.2 The United Kingdom (UK) reports a high level of disease, with the latest figures suggesting an estimated 850,000 people living with a diagnosis of dementia.3 With the population share of later-life age groups above the age of 65 years predicted to increase to 19.8 million over the next 50 years,4 the age-related disease burden is on the rise, including the challenges presented by dementia. The prevalence is predicted to reach over two million people by the year 2051, which will significantly add to the existing economic burden of dementia which is estimated at £26.3 billion per annum to the national economy.3
A concurrent shift in the oral health profile of older adults means that people are also retaining more of their natural teeth. The last published Adult Dental Health Survey within the UK, conducted in 2009, reported that 53% of adults over the age of 85 years have retained an average of 14 natural teeth.5 Also, the volume of ‘edentate’ adults had fallen to 6%, which represents an all-time recorded low.6 Therefore, the link between dementia and oral health is of significant concern in an ageing society, as good oral health is an essential component of active ageing, social participation, communication and general wellbeing.7
Research, albeit limited in volume, suggests that dementia is associated with worse oral health, although a number of recent systematic reviews highlight that the nature and direction of association is still unclear.8,9 There is greater awareness of the oral health challenges related to dementia, including tooth loss, periodontal disease risk, caries risk and increased prevalence of orofacial pain.10 Furthermore, increasing evidence also highlights a relationship between oral health conditions, wider negative health and frailty in people diagnosed with dementia, associated with systemic inflammatory responses, medication use, dietary changes and malnutrition.11 However, similar to people with other neurodegenerative disorders or disability, people with dementia are at a reduced capacity to maintain their oral health. The loss of cognitive and motor functions restricts their ability to take care of their oral and general health.12 Also, access to regular dental services and professional care is increasingly challenged, due to a rapid decline in health as dementia progresses.13 Issues unique to dementia such as, communication barriers, resistance, and behavioural difficulties further restrict the type of care that can be delivered.14 Therefore, people living with dementia are at a risk of poorer oral health and they present high treatment needs, yet timely care access and type of care they receive is more restricted than the general population.
While the evidence on dementia-related oral health challenges is growing, studies so far have mostly been conducted in residential facilities, despite the fact that most people with dementia live in the community.15 Older people who are no longer able to live independently are often admitted in to formal care facilities such as nursing homes, at which stage, they frequently present with poorer oral health and extensive dental care needs.16 However, limited information is available about the unique experiences of those people living in the community with a dementia diagnosis (including their carers) to understand how they view their health and what they do to manage their oral and dental health. Moreover, considering that oral disease is largely preventable and oral health can be maintained through self/home care management and routine clinical care, the lack of community-level focus presents missed opportunities to improve the lives of people with dementia. An understanding of the perspectives of community dwellers with dementia is, critical to support timely, person-centred care that is most helpful to them in their dementia journey. It is equally important to acknowledge the views and voices of the carers alongside people with dementia support a shared decision-making process.17 As research on the perspectives of community dwellers with dementia regarding their oral health is emerging, a synthesis and review of evidence are thus, required. Therefore, this rapid review was conducted to answer the following key questions:
-
1.
What do older adults, diagnosed with dementia (and their carers) do to look after their oral health?
-
2.
What are their beliefs and views about oral health/oral health care?
-
3.
What are their oral health-related experiences?
-
4.
What changes occur over time in their dementia journey?
The aim of this review is, therefore, to review existing evidence on oral health practices, beliefs/views and experiences of community-dwelling older adults living with dementia including their carers.
Materials and methods
Protocol and registration
This study has been conducted systematically according to Khangura’s methodology18 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19 Rapid reviews follow the process of systematic reviews with methodological adjustments for an accelerated assessment of the latest evidence available.20 Our review included a number of verified adaptations to a systematic review approach (including searching key databases, study screening by paired two groups, data extraction as a dyad and completion of review in a narrower timeframe), whilst using the systematic and transparent methodology to identify, screen, appraise, and analyse evidence from relevant studies. The protocol for the study is registered under the International Prospective Register of Systematic Reviews (ID ref: CRD42020213431).
Eligibility criteria
The full eligibility criteria for inclusion are described in Table 1a.
Search strategy and search terms
The search strategy was informed by library support services at King’s College London. Six databases were searched including Pubmed, Web of Science, OVID (Embase, MEDLINE [R] and PsycINFO) and Google Scholar. Searches were carried out in October 2020 and updated on 28 July 2021.
The search strategy included both keywords and National Library of Medicine’s MeSH (Medical Subject Headings). Given the limited research evidence in the field, broad search terms, identified in consultation with the library services/librarian, were necessary to capture evidence on the research questions. Search terms were combined using ‘OR’ and different groups of the PICO framework21 were combined using ‘AND’ for the final search outcome (Table 1b). Limits were applied to capture all relevant studies published in the last 10 years and in English language. In addition, manual citation tracking (backwards and forwards) for all included studies was conducted to identify additional relevant publications.
The search strategy was piloted ad refined in consultation with library services to ensure that key relevant papers were included.
Screening and study selection
Screening of the articles retrieved was conducted in three stages: removal of duplicates, screening of title and abstracts and full-text screening. The screening strategy was piloted among reviewers to maintain consistency against the set eligibility criteria.
Titles and abstracts of all included studies were scanned by four reviewers in two pairs (SK, MA, SC, and AGP). Full texts obtained for the studies that met the inclusion criteria were then screened in duplication (SK and MA), with disagreements resolved by consensus within the research team. Clarification of ambiguous findings and/or incomplete data was sought by contacting the first authors of publications.
Data extraction
Data extraction was carried out independently by two reviewers (SK and MA) using a customised data extraction table developed a priori and pilot tested for refinement. Differences were resolved in discussion or, if necessary, in consultation within the wider research team. Comparative data on participants without a dementia diagnosis and/or from non-community-dwelling residential settings were extracted for comparison, where available.
Methodological quality assessment
The quality of all included studies was assessed independently and in duplication by two reviewers (SK and MA) using the Mixed Methods Appraisal Tool (MMAT), 2018.22 MMAT uses quality scoring criteria for five different study designs (i.e., qualitative, quantitative randomised control trials, quantitative non-randomised, descriptive, mixed) with approximately five criteria per study design category. It is validated for use and assessment of the quality, reliability and risk of bias across qualitative and quantitative, as well as mixed methods studies.23
Data synthesis and analysis
Data were synthesised using a narrative disclosure given the heterogeneity of study design and data. Key characteristics of the studies are presented as an overall group (e.g., study year, date, country). Data are presented as subgroups pertaining to participant type (e.g., people with dementia, dementia type etc.), recruitment settings, and outcomes measured. Subgroup categorisation also includes information regarding key outcomes (beliefs/views, practices, experiences). It also includes analysis of longitudinal data and trends with regards to their dementia experience.
Results
Study selection
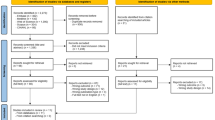
The electronic search resulted in 10,789 citations. Duplicates were removed to retain 8305 articles for abstract screening and an additional eight papers were identified from reference list screening. A total of 57 articles was shortlisted for full-text evaluation, of which, 16 met the defined inclusion and exclusion criteria and were retained for evaluation. Three additional papers were identified from the citation tracking, which resulted in a total of 19 papers representing 18 studies. The use of broad search terms to capture the breadth of research in the field while maintaining the minimum number of papers yielded many studies which did not meet the objectives of our review and were excluded during the initial screening. A PRISMA flow chart detailing the process of identification, database source, inclusion and exclusion of the studies is presented in Fig. 1.
Study characteristics
Eighteen studies and 19 papers were included in the review, published between 2010 and 2020.24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 Key study characteristics are presented in Table 2a. Seventeen papers were quantitative, which included six cross-sectional studies,25,28,29,30,33,35 two cross-sectional surveys,31,34 four cross-sectional, case-control studies,26,38,40,42 three case-control studies,32,39,41 two population-based cohort studies,33,36 one service evaluation,37 and one prospective study.24 Whilst two studies reported a mixed-method approach for data collection,25,27 only quantitative data were reported in the papers.
Across all included studies, the sample size ranged from 32,35 to 34,037.36 The papers presented a wide geographical representation with four studies from the United States of America (USA),28,29,34,41 and two in Hong Kong.32,38 Three studies across four publications were from Brazil,35,39,40,42 and one each from China,40 Finland,32 Ecuador,31 Thailand,27 Turkey,24 Italy,30 Sweden,36 and England.37
Participants were recruited from a range of community settings including those from dementia networks,26,35,40,42 university hospitals or public health institutes,24,30,39 daycare centres,29,38 memory assessment clinics,27,37 national registry or local communities,25,28,31,33,34,36 or a combination of the above. 41 Out of the four studies conducted in Brazil three,35,40,42 included participants from the same community settings, with two papers that included the same group of participants.40,42 The papers did not detail the living arrangements sufficiently to distinguish between older adults living in their own homes or in supportive housing facilities.
Fourteen papers directly included older adults with a dementia diagnosis and only five studies included caregivers in various capacities,26,29,35,37,39 one of which involved formal caregivers, as represented by nurses from Memory Assessment Clinic.37
Whilst a majority of studies used standardised tools to measure certain aspects of oral health status, there was limited information collected on oral health beliefs/views, care experiences or self-care practices. Dental caries was the most frequently recorded clinical disease measure, which was assessed using the DMFT (Decayed, Missing, and Filled Teeth) index,43 whilst oral hygiene, gingival bleeding, plaque levels and pocket depth were used to assess periodontal diseases. The International Statistical Classification of Diseases and Related Health Problems (ICD-10) and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) were the most common indicators of dementia diagnosis, whereas, the Mini-Mental State Examination (MMSE) tool,44 was the most common proxy measure of cognitive decline. Quality of life was most frequently reported using the General Oral Health Assessment Index (GOHAI).
Study participants
The age range of participants with dementia ranged from 72 to 82 years old, most of whom were female. Information regarding the type and severity of dementia in study participants was reported in most studies, but with varied detail (Table 2a).
Oral and dental disease
Most of the studies were clinically focused and reported on the oral and dental disease status of those with dementia.24,25,26,27,29,31,32,33,34,35,36,38,41
Generally, across all studies, the findings suggest that people with dementia had worse oral and dental health compared to those without dementia or their carers. The only exception to this was the study by Srisilapanan and Jai-Ua,27 where the provision of frequent dental appointments was made for older adults attending memory services for dementia. Access to dental care had a significant influence on oral health improvement for this study population, who presented with a similar DMFT score to the national average. Therefore, the fact that health systems for those with dementia had made dental care accessible, their oral health was not worse than the general population.
Generally, the absence of teeth, periodontal disease risk and dental caries were common. Luo et al.33 found that people with dementia had more teeth missing than those with moderate or no cognitive impairment, even after adjusting for confounders. While Gao et al.38 found no significant difference in some of the periodontal disease parameters (i.e., gingival bleeding, periodontal pockets and loss of attachment), the dementia group still had significantly higher levels of visible plaque and caries compared to those without dementia. Similarly, Del Brutto et al.31 reported that oral health was worse in people with dementia, which was directly correlated to the number of missing teeth. People with more than ten missing teeth presented with worse oral health. Arajulo et al.,39 looked at both number of missing teeth and periodontal disease status in older adults with dementia and found that this group had worse periodontitis and more missing teeth compared to their carers. They also frequently reported discomfort while chewing. Similar findings were reported by Campos,40 who showed that people with dementia had a decreased masticatory performance, which was negatively corelated to their cognitive function. The study found that those with more severe dementia-related cognitive decline, also had a lower masticatory function, which impacted their chewing capacity. Therefore, older adults with dementia, even at early stages of cognitive decline, appear to be at a higher risk of oral health deterioration.
The use removable prosthesis like dentures to replace missing tooth can facilitate improvement of oral functions, depending on how they are used and maintained. Although, denture use patterns in older adults with dementia was explored in only a few studies, an inconsistent pattern of denture use and an overall poor denture hygiene was reported. The study by Campos et al.35 found that people with dementia frequently wore dentures in both arches. However, Srisilapanan and Jai-Ua27 showed that despite presenting with least one missing tooth, less than half of participants wore dentures. In those who did wear a removable prosthesis, Campos et al.35 noted that the quality of the dentures used was very poor. In fact, Hatipogulu et al.24 reported that people with dementia were more likely to experience issues such as stomatitis, which was due to poor hygiene practices, including not removing dentures at night.
In addition, Chen et al.41 reported that people with cognitive impairment, including dementia, had more carious teeth and retained roots compared to those with normal cognitive function. Similar findings were also reported by Chu et al.32 who found that people with AD presented with more carious teeth compared to controls with normal cognition. Although most of the cases reported by Chu et al.32 had healthy oral mucosal status, their mean unstimulated salivary flow rate was significantly lower compared to controls. Campos et al.42 also reported that salivary flow rate was lower in the AD group than in the control group. Reduced salivary flow, dry mouth and use of xerostomic medications were shown to present a significant risk of deterioration of oral health as evidenced by Lexomboon et al.36 who found that the use of xerostomic medication, even up to 3 years prior to a dementia diagnosis, increased the need for tooth extraction. However, no significant associations between xerostomic medication use and the need for dental restorations or preventive procedures were found.
In terms of oral and dental health status of those with different types of dementia, Syrjala et al.25 showed that people with AD had more teeth than those with vascular dementia or other types of dementia. They also presented with poorer oral hygiene, as well as denture hygiene, compared to other dementia groups. However, the results do not allow clear estimations of how these parameters relate to community dwellers with different types of dementia as only half of the total participants recruited lived in community settings, with the rest in formal/institutional care.
In terms of decline in oral health in relation to dementia stage, Ribeiro et al.26 showed that those with advanced AD had more calculus and biofilm compared to people with milder dementia or no dementia. Interestingly, Srisilapanan et al.27 showed that dental caries was highest in the moderate dementia group, in comparison those in mild or severe disease stages. Although the authors attributed increased care support for those in severe dementia stage for the observed improvement in dental caries status, we could not find any evidence on level of care in relation to dementia decline and oral health outcome.
Studies exploring dental health of people with dementia across different residential settings in comparison to those living in the community, presented limited information on varied patterns of denture use and the number of missing teeth.29 In general, more nursing home residents, with one and eight teeth, wore removable partial dentures compared to community dwellers. However, the pattern was reversed in those with more teeth remaining, with more community dwellers reporting partial denture use compared with nursing home residents.29
Overall, evidence suggests that older adults with dementia have higher risk of dental caries, tooth loss and periodontal disease, which impacts their capacity to chew and consume food. Although a varied pattern of denture use to replace masticatory function was noted, the prosthesis were of poor quality, with a reported lack in denture hygiene practice. Further oral and dental health issues, such as, reduced salivary flow and the risk of dental extractions due to xerostomic medication use were also noted.
There was limited evidence to support the view that people with Alzheimer’s dementia had worse oral and dental health, compared to those with other dementia types. Also, a varied risk of oral and dental health deterioration with respect to the dementia severity and a difference in oral health behaviour in relation to residential settings were noted. Even though, more research is needed on difference in oral health risk in relation to dementia severity and residential arrangements, a generally poor oral and clinical dental health status was reported in the studies we reviewed.
Quality assessment
Using the MMAT tool guidance, an overall quality score was given for each study. Although two studies reported using a mixed-method approach,29,31 neither provided information on qualitative methods used or data collected. Therefore, we assessed these papers as quantitative.
Only one,38 out of 19 papers met all of the five quality criteria scoring a 5* rating. The remaining studies were largely 3* 24,27,32,35,36,39,41 or 4* 28,31,33,34,40,42 with the remainder scoring 2* 26,30,37 or below.25,29 Details of the quality assessed using the MMAT criteria are presented in Table 3.
Key findings
The major findings presented across all included studies were explored in relation to our research questions about oral health-related practices, beliefs and views, experiences and changes over time of community-dwellers with dementia. Key findings are summarised in Table 2b.
What do older adults with dementia and their carers do to look after their oral health?
Only seven studies24,27,29,32,33,38,41 provided information regarding oral health-related practices in those living with dementia. Information regarding tooth brushing habits, use and care of dental prostheses and care assistance required were obtained from the studies.
Daily oral care (with or without assistance)
Studies showed that older adults with dementia living in their own homes with community interactions presented with poor oral hygiene practice—with reported difficulties in their ability to self-perform daily oral care and so they needed care-assistance. Dementia-related cognitive and functional issues including forgetfulness, unwillingness to brush, as well as lack of dexterity were identified as the difficulties people with dementia faced in performing daily oral hygiene practice.32
Similarly, Gao et al.38 found that only 3% of the participants with dementia brushed their teeth daily due to difficulties with tooth brushing. In comparison, 100% of controls without dementia stated that they brushed their teeth every day. Also, the number of people requiring care assistance was higher in the dementia group compared to controls. Although, Srisilapanan and Jai-Ua27 reported that a majority of people with dementia were functionally independent, with normal hand and arm functions, only half of them were able to perform oral care by themselves. The evidence was further supported by Chen et al.41 who found that community dwellers with cognitive impairment including dementia had a greater need for carer assistance to maintain their oral hygiene, which was directly related to their risk of dental caries or retained roots. In fact, the loss of ability to self-perform oral hygiene significantly increased the risk of poor oral health in these groups, reinforces the need for improved carer support.
Similar findings were also reported in an earlier study by Chen et al.29 when comparing people with dementia living in different care settings. Even though community dwellers demonstrated better capacity for oral hygiene practice compared to those in assisted living or nursing homes, participants living in community required supervision, while some were non-cooperative to carer support. In one population-based study by Luo et al.33 it was found that older adults with dementia scored significantly higher on Activity of Daily Living Scale (ADL) compared to people without dementia or those with mild cognitive impartment, indicating a greater need for care and assistance for the dementia group. In fact, Hatipoglu et al.24 showed that low cognitive function was more strongly related to oral and dental practices than reduced functional assessment scores.
Therefore, regardless of their functional independence or residential arrangements, people with dementia had poor oral hygiene practices and relied on caregiver assistance to look after their oral and dental health. The loss in cognitive function due to dementia was found to be a more significant predictor of poor oral health practice than their functional abilities.
What are their beliefs and views about oral health/oral health care?
Although eight studies26,28,30,31,34,35,37,39 provided information on the views and beliefs of those with dementia, they were mostly focused on understanding their self-perceived quality of life in relation to their oral and dental status. However, there was a lack of evidence on their perception about the importance of oral health care and areas of care needs.
Self-perceived oral health and oral health-related quality of life
Across the five studies26,28,30,35,39 that assessed self-perceived Oral Health Related Quality of Life (OHRQoL) in community residents with dementia, the evidence varied. Lee et al.28 found that people with mild dementia viewed and reported their quality of life as significantly lower than participants with normal cognitive function, even after controlling for confounders. Ciccu et al.30 identified issues such as difficulty in speaking, self-consciousness due to oral health problems, concerns about their oral status and limiting contact with people because of the condition of oral health were the most significant factors which influenced how people with dementia viewed their quality of life. In addition, clinical parameters of periodontal disease destruction, such as gingival bleeding and increased probing depth, were also identified as factors that negatively impacted those with dementia, who reported as being dissatisfied about their oral health.30 Furthermore, Lee et al.28 found that in people with mild dementia, higher GOHAI scores representing better self-perceived oral health, were correlated with better clinical disease status. In particular, the total number of decayed root surfaces was the most significant correlate of the participants’ oral health perception, with those with more decayed coronal surfaces reporting lower GOHAI total scores. Therefore, we found that when asked to subjectively evaluate their health and wellbeing, people with dementia reported a number of clinical, functional and aesthetic factors related to their oral health, which negatively influenced their quality of life.
In contrast, in four, albeit smaller studies, there was evidence that people with dementia had better perception about their oral health, and oral health-related quality of life.26,35,39,42 Ribeiro et al.26 found that people in severe stages of dementia scored higher on GOHAI index, indicating that they had a more positive view of their oral health. Although, this group also viewed their quality of life as similar to those without dementia, they were more reliant on their caregiver support and required assistance in answering questions. Similarly, Araujo et al.39 found that despite having fewer teeth and more severe periodontitis, people with mild or moderate AD reported more positive GOHAI scores when compared with their family members without dementia. Furthermore, Campos et al.42 found that when measuring masticatory functions, people with AD consistently reported higher GOHAI scores despite a compromised masticatory efficiency. These findings raise important issues about the capacity of older with dementia to correctly identify and communicate their needs. The fact that despite poor oral health and dental disease status, people with dementia seemed less aware of these issues, raises concerns about missed opportunities to address undetected and often preventable oral health problems.
Interestingly, Campos et al.35 noted that caregivers also had similar views about the quality of life of those they were caring for, and they too reported it as more favourable than clinically verified. Although, the total GOHAI score and score for psychological domain showed high inter-rater agreement between patient and caregiver, domains such as speaking, biting and food consumption difficulties were less strongly correlated. Therefore, the caregiver group was also less aware of the range of oral health issues present.
Self-rated appearance in relation to their oral health
Only one study reported on the views of people with dementia about their oral health appearance.39 It showed that people in early stages of dementia generally viewed their appearance as good, although this changed as their dementia progressed. However, information about how this compares to the control group, which included their family members, was not available.39
Self-perceived social support
Lee et al.34 highlighted that people with dementia perceived their social network as weaker and they also had lower confidence in their social network compared to those without dementia.
What are their oral health-related experiences?
Experience of accessing professional care
Only two studies provided an insight into the experience of older adults diagnosed with dementia in terms of accessing professional care. An American based study by Lee et al.34 reported that nearly half of all participants with dementia had no regular schedule of attending a dentist, which was lower compared to of those without dementia. This was also affected by the fact that most people with dementia did not have dental insurance to cover the treatment costs. In contrast, a study conducted in England by Emanuel and Sorensen37 found that despite a dementia diagnosis, older adults viewed their oral health as important and were keen on continuing their professional care. In fact, most of participants with dementia regularly attended a dental practice and about half of all patients attended for regular hygienist sessions. However, those who did attend professional services did not always receive routine preventative care and advise including fluoride supplement and dietary or oral hygiene advice. It is worth noting that the two studies that reported on professional care attendance were conducted in England and the USA, which have different healthcare systems and social support provisions. Although we are unable to draw direct comparisons or robust conclusions due to small sample size and heterogeneity, further research in the field is required to understand how care attendance could be implicated by social and financial support for those requiring care. Nevertheless, the evidence from the study in the UK shows a clear scope to improve prevention and care for people with dementia, even in countries with more facilitative social care systems.
Changes over time
Only one study36 reported on changes over time and did so in relation xerostomic medication and dental health. Although, the change was assessed in relation to progression of dementia, they found that use of xerostomic medication used prior to a diagnosis of dementia (up to 3 years) resulted in poorer oral health in a longitudinal dose-response manner.
Discussion
One of the most notable findings of this rapid review is the lack of robust research evidence on the oral health-related views/beliefs, self-care or assisted care practices, as well as experiences of professional care of community dwellers with dementia. This paucity of evidence was reflected in our screening process, whereby only 19 out of the 57 full texts reviewed met our criteria. The main reasons for exclusion were lack of clarity regarding inclusion of participants with dementia, with studies recording a dementia diagnosis, but then excluding the participants from data collection. These issues are not unique to our study as highlighted by earlier systematic reviews, which found that people with cognitive decline and dementia are frequently excluded from research, due to often poorly justified concerns surrounding safeguarding issues and ethical challenges.45,46 A move towards more flexible research approach and adaptive study consent strategies have made way for greater research inclusivity,47 which also need to be reflected in practice. Also, as most of the studies we reviewed were cross sectional or case-control, we note a lack of longitudinal data on oral health change in relation to dementia progression. Observational studies that are cross-sectional or case-control in design allow collection of data at a single point in time as a snapshot overview. However, it does not provide information about changes over time to be captured, as allowed by a longitudinal study design. This may be an important consideration for studies involving those with neurodegenerative disorders including dementia, which can rapidly progress within a short timeframe. Moreover, normative data collected through quantitative methods need to be supplemented by in depth exploration of the perspectives of those with dementia, to support an overall picture of their oral care needs and explore any dissonance with reported health-related quality of life. In this regard, we recommend future studies to consider using a longitudinal, mixed research methods to fully capture the psycho-social aspects of oral health care and changes over time for those with a clinical diagnosis of dementia. Studies should also consider including the views and experiences of their carers, both formal and informal.
Although most of the studies we reviewed were clinically focused, they did however confirm that community dwellers with dementia generally have poor professionally assessed oral health (notably missing teeth, dental caries and periodontal disease risk). To maintain good oral and dental health, daily tooth brushing, limiting sugary diet, using fluoride supplements, as well as, removal and cleaning of dentures are essential.48,49 Our findings on oral health practices, where available, showed that most people with dementia do not brush their teeth daily and they are less able to look after their dentures due to cognitive and functional challenges. The loss of cognitive function, even in mild stages of dementia, may present issues with language, memory, attention, and apraxia, which hinders their ability to self-perform oral care.50 In addition, physical impairments, including a gradual decline in manual dexterity and motor skills also impacts their ability to perform oral and personal self-care.51 A generally poor clinical disease status (which we noted, regardless of dementia type, severity and community residential setting) is an important indication of substandard oral care practices and a reduced capacity for self-performed care. This is unsurprising, given our findings, which suggested that even those who were functionally independent required caregiver supervision. Therefore, a major influence on the oral health practices of those with dementia is shaped by their reliance on their caregivers, who may not have the necessary skills, knowledge and training to provide appropriate oral health care.52,53 However, the lack of information regarding the level and type of caregiver support that is available to these community dwellers limits our overall understanding of their daily self-care and assisted care practices to maintain their oral health.
What people with dementia and their carers do to take care of their mouth can inadvertently be affected by how they view their oral health and perceive its impact on their quality of life. The conflicting findings in self-reported oral health and clinically verified dental status, across the different studies we reviewed, raise important concerns about the ability of people with dementia to recognise and communicate their oral care needs. Issues such as progressive memory loss and personality changes are unique to those with dementia and can have an effect on how they view their health and wellbeing.54 Furthermore, as dementia progresses, individual preferences and perspectives can be harder to determine due additional communication and behavioural barriers.55 Therefore, it is important to raise awareness of the importance of oral health care and provide routine professional care for people living with dementia. It is equally important to ensure that their primary caregiver or family members are adequately supported to identify early signs of problems and arrange suitable clinical care.
As a proactive approach, integrating dialogues about oral health care at the earliest signs of cognitive decline means that effective care pathways can be established while people are able to partake in making decisions about their own health and health care. Effective intervention plans will also require training carers with a goal of promoting and maintaining health; not just the oral health of vulnerable groups, but also the overall general health and wellbeing of those people for whom they care.56,57 This may be significant for those living in their own homes and communities while being looked after by informal caregivers, mostly spouses and children, who need additional professional support and advice on how to direct timely care across dental care pathways.57
Access to dental services is an important concern as dental professionals are uniquely placed to create, and maintain long-term relations with the patients they care for, providing opportunities to have meaningful discussions about the dental care provisions for later disease stages.55 Dental care professionals also have an important role of engaging and instructing carers alongside those with dementia to implement effective oral care routine in consideration of patient wishes and care needs.48 It is therefore important to understand how community dwellers and their carers engage with professional services and what services are made available to them. Although this rapid review highlights a lack of emphasis on professional care access, one study did provide evidence that where dental services were offered as a part of routine geriatric care, patients’ oral health status was better than the national profile.27 Recent systematic reviews have also reinforced the need for timely professional care using both subjective and objective measures of health assessment, in order to capture the range of an age-related loss of oral health function due to cognitive and functional decline in people with dementia.58,59
Furthermore, we also note an equity dimension to care access influenced by the socio-economic structures and payment models for those with dementia, which could translate into care access behaviour. Although, there are methodological and statistical differences across studies we reviewed, which do not allow a direct comparison, these findings do raise important questions about health system issues and socio-economic barriers affecting professional care access. This resonates with a recent research paper from our research group which highlights similar challenges and reinforces the need for efficient integrated systems that provide a clear pathway for people with dementia to access and navigate care services.60 Therefore, useful signposts to dementia friendly dental services, if available, would be of significant advantage to improve access to professional support. Also, existing health care systems should be reviewed to provide necessary training and support for the dental workforce who are challenged with the critical task of supporting the health of the most vulnerable people in society.60 Further research and action in this field are urgently required.
Strengths and limitations
This review has several strengths, which should be acknowledged. First, the review process was conducted by a multidisciplinary team containing researchers, dental and care professionals, and public health expertise. Second, to the best of our knowledge, this is a first review in a high priority area with emerging research evidence. Therefore, the review provides helpful directions and suggestions for future studies in the field.
We also acknowledge the limitation of our search strategy that resulted in capturing a large volume of studies. The strategy was refined in consultation with the library services to ensure that the minimum number of papers was identified whilst gaining maximum coverage to address the research questions. Furthermore, whilst it could be argued that the restriction to the 10-year period may have excluded any earlier papers on the subject, the backwards/forwards search did not reveal any further early data for the review. In addition, inclusion of three papers from the same author group and two from the same study may have introduced bias, which may implicate the generalisability of findings.
Conclusion
Although there was evidence on the oral health care needs of those living with dementia in their communities, there was limited evidence on their oral health-related practices, beliefs/views and experiences. The available evidence suggests that the oral and dental health of this population is generally poor and their ability for self-care (notably oral hygiene) reduces with cognitive decline. With regards to self-perceived oral health and quality of life, the evidence varied, with noted discrepancies in relation to their normative care needs. Few studies also reported a low self-perception in people with dementia, who also viewed their social support as weak. Research evidence, albeit weak, suggests a reduced capacity of people with dementia and their carers to correctly identify and report oral health-related issues experienced by this population. There was a paucity of evidence on dental care experience with dental access appearing system dependent. Only one study investigated changes over time and highlighted an increased risk of oral health deterioration in relation to the use of xerostomic medication use.
Overall, the limited evidence on perspectives of a largely underrepresented population group with clear oral health care needs highlights an urgent need for more methodologically robust research in the field. Further studies should consider mixed-method approaches, to capture multiple perspectives of people with dementia and to bring their voice to the forefront. Also, longitudinal studies would be useful to capture the unique lived experiences of people living with dementia and identify how they change over time.
Change history
14 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41405-021-00092-3
References
World Health Organisation. 10 facts on dementia. 2019. https://www.who.int/features/factfiles/dementia/en/#:~:text=The%20total%20number%20of%20new,and%20152%20million%20in%202050.
Alzheimer’s Society. Dementia UK report. 2020. https://www.alzheimers.org.uk/about-us/policy-and-influencing/dementia-uk-report.
The National Institute for Health and Care Excellence. Dementia: what are the risk factors? 2020. https://cks.nice.org.uk/topics/dementia/background-information/risk-factors/.
Office of National Statistics. Overview of the UK population: January 2021. 2021.https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/january2021.
NHS Digital. Adult Dental Health Survey. 2009. https://digital.nhs.uk/data-and-information/publications/statistical/adult-dental-healthsurvey/adult-dental-health-survey-2009-summary-report-and-thematic-series. [Accessed 28th March 2021].
Steele JG, Treasure ET, O'Sullivan I, Morris J, Murray JJ. Adult Dental Health Survey 2009: transformations in British oral health 1968–2009. Br Dent J. 2012; 213:523–7. https://doi.org/10.1038/sj.bdj.2012.1067.
World Health Organisation. Active ageing: a policy framework. 2002. http://whqlibdoc.who.int/hq/2002/WHO_NMH_NPH_02.8.pdf.
Wu B, Fillenbaum GG, Plassman BL, Guo L. Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc. 2016;64:739–51.
Tonsekar PP, Jiang SS, Yue G. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. 2017;34:151–163.
Foley NC, Affoo RH, Siqueira WL, Martin RE. A Systematic Review Examining the Oral Health Status of Persons with Dementia. JDR Clinical & Translational Research. 2017;2:330–42. https://doi.org/10.1177/2380084417714789.
Dibello V, Lozupone M, Manfredini D, Dibello A, Zupo R, Sardone R, et al. Oral frailty and neurodegeneration in Alzheimer’s disease. Neural Regen Res. 2021;16:2149–53. https://doi.org/10.4103/1673-5374.310672.
British Dental Association. BDA evidence summary: dental problems and their management in patients with dementia. 2013. https://bda.org/searchresults?k=dental%20problems%20and%20their%20management%20in%20patients%20with%20dementia.
Göstemeyer G, Baker SR, Schwendicke F. Barriers and facilitators for provision of oral health care in dependent older people: a systematic review. Clin Oral Investig. 2019;23:979–93.
Chalmers JM. Behavior management and communication strategies for dental professionals when caring for patients with dementia. Spec Care Dent. 2000;20:147–54.
Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Update Second Edition. 2014. http://eprints.lse.ac.uk/59437/1/Dementia_UK_Second_edition_-_Overview.pdf.
Karki A, Monaghan N, Morgan M. Oral health status of older people living in care homes in Wales. Br Dental J. 2015;219:331–4. https://doi.org/10.1038/sj.bdj.2015.756.
Fazio S, Pace D, Flinner J, Kallmyer B. The Fundamentals of Person-Centered Care for Individuals With Dementia. Gerontologist. 2018;18:S10–S19. https://doi.org/10.1093/geront/gnx122.
Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst. Rev. 2012;1:10.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–9.
Public Health England. UK NSC evidence review process. Appendix F: requirements for UK NSC evidence summaries. 2015. https://www.gov.uk/government/publications/uk-nsc-evidence-review-process/uk-nsc-evidence-review-process#fn:1.
Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–3.
Hong QN, Pluye P, Fabregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT) Version 2018 user guide. 2018. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf.
Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49:47–53.
Hatipoglu MG, Kabay SC, Güven G. The clinical evaluation of the oral status in Alzheimer-type dementia patients. Gerodontology. 2011;28:302–6.
Syrjälä A-MH, Ylöstalo P, Ruoppi P, Komulainen K, Hartikainen S, Sulkava R, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29:36–42.
Ribeiro GR, Costa JL, Ambrosano GM, Garcia RC. Oral health of the elderly with Alzheimer’s disease. Oral. Surg. Oral. Med. 2012;114:338–43.
Srisilapanan P, Jai-Ua C. Oral health status of dementia patients in Chiang Mai Neurological Hospital. J Med Assoc Thai. 2013;96:351–7.
Lee KH, Wu B, Plassman BL. Cognitive function and oral health-related quality of life in older adults. J Am Geriatr Soc. 2013;61:1602–7.
Chen X, Clark JJ, Naorungroj S. Oral health in older adults with dementia living in different environments: a propensity analysis. Spec Care Dent. 2013;33:239–47.
Cicciù M, Matacena G, Signorino F, Brugaletta A, Cicciù A, Bramanti E. Relationship between oral health and its impact on the quality life of Alzheimer’s disease patients: a supportive care trial. Int J Clin Exp Med. 2013;6:766–72.
Del Brutto OH, Gardener H, Del Brutto VJ, Maestre GE, Zambrano M, Montenegro JE, et al. Edentulism associates with worse cognitive performance in community-dwelling elders in rural Ecuador: results of the Atahualpa project. J Community Health. 2014;39:1097–100.
Chu CH, Ng A, Chau AMH, Lo ECM. Oral health status of elderly chinese with dementia in Hong Kong. Oral Health Prev Dent. 2015;13:51–57.
Luo J, Wu B, Zhao Q, Guo Q, Meng H, Yu L, et al. Association between tooth loss and cognitive function among 3063 Chinese older adults: a community-based study. PLoS ONE. 2015;10:e0120986.
Lee KH, Wu B, Plassman BL. Dental care utilization among older adults with cognitive impairment in the USA. Geriatr Gerontol Int. 2015;15:255–260.
Campos CH, Ribeiro GR, Rodrigues Garcia RC. Oral health-related quality of life in mild Alzheimer: patient versus caregiver perceptions. Spec Care Dentist. 2016;36:271–276.
Lexomboon D, Tan ECK, Hoijer J, Garcia-Ptacek S, Eriksdotter M, Religa D, et al. The effect of xerostomic medication on oral health in persons with dementia. J. Am. Med. Dir. Assoc. 2018;9:1080–1.
Emanuel R, Sorensen A. A. study of oral health prevention behaviours for patients with early stage dementia. Br Dent J. 2018;224:38–42.
Gao SS, Chen KJ, Duangthip D, Lo ECM, Chu CH. The Oral Health Status of Chinese Elderly People with and without Dementia: A Cross-Sectional Study. Int J Environ Res Public Health. 2020;17:1913. https://doi.org/10.3390/ijerph17061913.
Araujo RD, Villoria GEM, Luiz RR, Esteves JC, Leao ATT, Feres E. Association between periodontitis and Alzheimer’s disease and its impact on the self-perceived oral health status: a case-control study. Clin Oral Investig. 2020;25:555–62.
Campos CH, Ribeiro GR, Costa JL, Rodrigues Garcia RC. Correlation of cognitive and masticatory function in Alzheimer’s disease. Clin Oral Investig 2017;21:573–8.
Chen X, Clark JJ, Chen H, Naorungroj S. Cognitive impairment, oral self-care function and dental caries severity in community-dwelling older adults. Gerodontology. 2015;32:53–61.
Campos CH, Ribeiro GR, Rodrigues Garcia RCM. Mastication and oral health-related quality of life in removable denture wearers with Alzheimer disease. J. Prosthet. Dent. 2018;119:764–8.
Roland E, Gueguen G, Longis MJ, Boiselle J. Validation of the reproducibility of the DMF Index used in bucco-dental epidemiology and evaluation of its 2 clinical forms. World Health Stat. Q. 1994;47:44–61.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–98.
Taylor JS, DeMers SM, Vig EK, Borson S. The disappearing subject: exclusion of people with cognitive impairment and dementia from geriatrics research. J. Am. Geriatr. Soc. 2012;60:413–9.
West E, Stuckelberger A, Pautex S, Staaks J, Gysels M. Operationalising ethical challenges in dementia research—a systematic review of current evidence. Age Ageing. 2017;46:678–687.
Webb J, Williams V, Gall M, Dowling S. Mis fitting the research process: shaping qualitative research “in the Field” to fit people living with dementia. Int J Qual Method. 2020;19:1–11. https://doi.org/10.1177/1609406919895926.
Marchini L, Ettinger R, Caprio T, Jucan A. Oral health care for patients with Alzheimer’s disease: An update. Spec Care Dent. 2019;39:262–273.
Public Health England. Delivering better oral health: an evidence-based toolkit for prevention.Third edition. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/605266/Delivering_better_oral_health.pdf.
Ghezzi EM, Ship JA. Dementia and oral health. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2000;89:2–5.
Barbe AG, Kupeli LS, Hamacher S, Noack MJ. Impact of regular professional toothbrushing on oral health, related quality of life, and nutritional and cognitive status in nursing home residents. Int J Dent Hyg. 2020;18:238–50.
Khanagar S, Kumar A, Rajanna V, Badiyani BK, Jathanna VR, Kini PV. Oral health care education and its effect on caregivers’ knowledge, attitudes, and practices: a randomized controlled trial. J Int Soc Prev Community Dent. 2014;4:122–8.
Reis SC, Marcelo VC, da Silva ET, Leles CR. Oral health of institutionalised elderly: a qualitative study of health caregivers’ perceptions in Brazil. Gerodontology. 2011;28:69–75.
Van de Rijt L, Weijenberg R, Feast AR, Vickerstaff V, Lobbezoo F, Sampson EL. Oral health and orofacial pain in people with dementia admitted to acute hospital wards: observational cohort study. BMC geriatrics. 2018;18:121. https://doi.org/10.1186/s12877-018-0810-7.
Geddis-Regan A, Kerr K, Curl C. The impact of dementia on oral health and dental care, Part 2: Approaching and Planning Treatment. Prim Dent J. 2020;9:31–37.
Prorok JC, Horgan S, Seitz DP. Health care experiences of people with dementia and their caregivers: a meta-ethnographic analysis of qualitative studies. CMAJ. 2013;85:E669–E680.
Manchery N, Subbiah GK, Nagappan N, Premnath P. Are oral health education for carers effective in the oral hygiene management of elderly with dementia? A systematic review. Dent Res J. 2020;17:1–9.
Dibell V, Zupo R, Sardone R, Lozupone M, Castellana F, Dibello A, et al. Oral frailty and its determinants in older age: a systematic review. Lancet Healthy Longev. 2021;2:e507–e520.
Ming Y, Hsu S-W, Yen Y-Y, Lan S-J. Association of oral health–related quality of life and Alzheimer disease: a systematic review. J. Prosthet. Dent. 2020;124:168–75.
Curtis SA, Scambler S, Manthorpe J, Samsi K, Rooney YM, Gallagher JE. Everyday experiences of people living with dementia and their carers relating to oral health and dental care. Dementia (London). 2021;20:1925–39. https://doi.org/10.1177/1471301220975942.
Acknowledgements
There were no external sources of funding for this work. We would like to thank library services team at King’s College London, for their assistance with the search terms and strategy. Also, a special thanks to Aphra Garner-Purkis for her help with designing the initial search terms and literature screening.
Author information
Authors and Affiliations
Contributions
SK, JEG and MA had oversight of the study planning and execution, and to the conception, design, data acquisition, synthesis and interpretation. SC and SS contributed to conception, design, data synthesis and interpretation. All authors critically revised the final manuscript and approved changes prior to publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
KC, S., Aulakh, M., Curtis, S. et al. Perspectives of community-dwelling older adults with dementia and their carers regarding their oral health practices and care: rapid review. BDJ Open 7, 36 (2021). https://doi.org/10.1038/s41405-021-00091-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-021-00091-4
This article is cited by
-
BDJ Open 2021 - our most successful year to date
British Dental Journal (2022)