Abstract
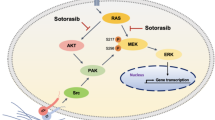
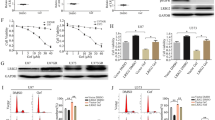
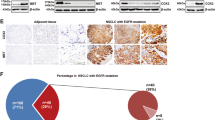
Although several KRasG12C inhibitors have displayed promising efficacy in clinical settings, acquired resistance developed rapidly and circumvented the activity of KRasG12C inhibitors. To explore the mechanism rendering acquired resistance to KRasG12C inhibitors, we established a series of KRASG12C-mutant cells with acquired resistance to AMG510. We found that differential activation of receptor tyrosine kinases (RTKs) especially EGFR or IGF1R rendered resistance to AMG510 in different cellular contexts by maintaining the activation of MAPK and PI3K signaling. Simultaneous inhibition of EGFR and IGF1R restored sensitivity to AMG510 in resistant cells. PI3K integrates signals from multiple RTKs and the level of phosphorylated AKT was revealed to negatively correlate with the anti-proliferative activity of AMG510 in KRASG12C-mutant cells. Concurrently treatment of a novel PI3Kα inhibitor CYH33 with AMG510 exhibited a synergistic effect against parental and resistant KRASG12C-mutant cells in vitro and in vivo, which was accompanied with concomitant inhibition of AKT and MAPK signaling. Taken together, these findings revealed the potential mechanism rendering acquired resistance to KRasG12C inhibitors and provided a mechanistic rationale to combine PI3Kα inhibitors with KRasG12C inhibitors for therapy of KRASG12C-mutant cancers in future clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging All KRAS mutants. Cancer Discov. 2022;12:924–37.
Khan I, Rhett JM, O’Bryan JP. Therapeutic targeting of RAS: New hope for drugging the “undruggable”. Biochim Biophys Acta Mol Cell Res. 2020;1867:118570.
Schaber MD, Garsky VM, Boylan D, Hill WS, Scolnick EM, Marshall MS, et al. Ras interaction with the gtpase-activating protein (Gap). Proteins. 1989;6:306–15.
Lu S, Jang H, Gu S, Zhang J, Nussinov R. Drugging Ras GTPase: a comprehensive mechanistic and signaling structural view. Chem Soc Rev. 2016;45:4929–52.
Banno K, Yanokura M, Iida M, Masuda K, Aoki D. Carcinogenic mechanisms of endometrial cancer: involvement of genetics and epigenetics. J Obstet Gynaecol Res. 2014;40:1957–67.
Siqi Li ABCMC. A model for RAS mutation patterns in cancers: finding the sweet spot. Nat Rev Cancer. 2018;18:767–77.
Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303:72–4.
Sukumar S, Notario V, Martin-Zanca D, Barbacid M. Induction of mammary carcinomas in rats by nitrosomethyl-urea involves the malignant activation of the H-ras-1 locus by single point mutations. Nature. 1983;306:658–61.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13:828–51.
Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–23.
Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS mutant cancers with a covalent G12C-Specific inhibitor. Cell. 2018;172:578–89.e17.
Johnson ML, Ou SHI, Barve M, Rybkin II, Papadopoulos KP, Leal TA, et al. Activity and safety of Adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Cancer. 2020;138:S2.
Jänne PA, Rybkin II, Spira AI, Riely GJ, Papadopoulos KP, Sabari JK, et al. Activity and safety of Adagrasib (MRTX849) in advanced/ metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer. 2020;138:S1–2.
Patricelli MP, Janes MR, Li L-S, Hansen R, Peters U, Kessler LV, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6:316–29.
Lito P, Solomon M, Li LS, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351:604–8.
Li H-y, Qi W-l, Wang Y-x, Meng L-h. Covalent inhibitor targets KRasG12C: a new paradigm for drugging the undruggable and challenges ahead. Genes Dis. 2021; https://doi.org/10.1016/j.gendis.2021.08.011.
Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63:52–65.
Jiao D, Yang S. Overcoming resistance to drugs targeting KRAS mutation. Innovation. 2020;1:100035.
Nakajima EC, Drezner N, Li X, Mishra-Kalyani PS, Liu Y, Zhao H, et al. FDA Approval Summary: Sotorasib for KRAS G12C-mutated metastatic NSCLC. Clin Cancer Res. 2022;28:1482–6.
Ou SI, Janne PA, Leal TA, Rybkin, II, Sabari JK, Barve MA, et al. First-in-human phase I/IB dose-finding study of Adagrasib (MRTX849) in patients with advanced KRAS(G12C) solid tumors (KRYSTAL-1). J Clin Oncol. 2022;40:JCO2102752.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus Crizotinib in Untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38.
Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Cancer Res. 2020;26:1633–43.
Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–39.
Misale S, Fatherree JP, Cortez E, Li CD, Bilton S, Timonina D, et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin Cancer Res. 2019;25:796–807.
Xue JY, Zhao Y, Aronowitz J, Mai TT, Vides A, Qeriqi B, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–5.
Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384:2382–93.
Liu XL, Wang BB, Wang Y, Wang YX, Yang CH, Tan C, et al. Unbiased screening reveals that blocking exportin 1 overcomes resistance to PI3Kalpha inhibition in breast cancer. Signal Transduct Target Ther. 2019;4:49.
Li X, Tong LJ, Ding J, Meng LH. Systematic combination screening reveals synergism between rapamycin and sunitinib against human lung cancer. Cancer Lett. 2014;342:159–66.
Shi JJ, Chen SM, Guo CL, Li YX, Ding J, Meng LH. The mTOR inhibitor AZD8055 overcomes tamoxifen resistance in breast cancer cells by down-regulating HSPB8. Acta Pharmacol Sin. 2018;39:1338–46.
Tang J, Wennerberg K, Aittokallio T. What is synergy? The Saariselka agreement revisited. Front Pharmacol. 2015;6:181.
Ni D, Li X, He X, Zhang H, Zhang J, Lu S. Drugging K-Ras(G12C) through covalent inhibitors: mission possible? Pharmacol Ther. 2019;202:1–17.
Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871–9.
Rodenhuis, S. ras and human tumors. Semin Cancer Biol. 1992;3:241–7.
Rubin SM, Sage J, Skotheim JM. Integrating old and new paradigms of G1/S control. Mol Cell. 2020;80:183–92.
Molina-Arcas M, Moore C, Rana S, van Maldegem F, Mugarza E, Romero-Clavijo P, et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci Transl Med. 2019;11:eaaw7999.
Xiang HY, Wang X, Chen YH, Zhang X, Tan C, Wang Y, et al. Identification of methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-morpholinopyrrolo[2,1-f][1,2,4]triazin-2-yl)-4-(trifluoromethyl)pyridin-2-yl)carbamate (CYH33) as an orally bioavailable, highly potent, PI3K alpha inhibitor for the treatment of advanced solid tumors. Eur J Med Chem. 2021;209:112913.
Adachi Y, Ito K, Hayashi Y, Kimura R, Tan TZ, Yamaguchi R, et al. Epithelial-to-mesenchymal transition is a cause of both intrinsic and acquired resistance to KRAS G12C inhibitor in KRAS G12C-mutant non-small cell lung cancer. Clin Cancer Res. 2020;26:5962–73.
Suzuki S, Yonesaka K, Teramura T, Takehara T, Kato R, Sakai H, et al. KRAS inhibitor resistance in MET-amplified KRAS (G12C) non-small cell lung cancer induced By RAS- and non-RAS-mediated cell signaling mechanisms. Clin Cancer Res. 2021;27:5697–707.
Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nature. 2018;15:709–20.
Hallin, J, Engstrom, LD, Hargis, L, Calinisan, A, Aranda, R, Briere, DM. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71.
Moore AR, Rosenberg SC, McCormick F, Malek S. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19:533–52.
Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316–25.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82104199, 82173832 and 81973345), the Lingang Laboratory (LG202103-02-03) and Science and Technology Commission of Shanghai Municipality (22ZR1474400).
Author information
Authors and Affiliations
Contributions
LHM, YXW, and WLQ conceived the study and wrote the manuscript. WLQ carried most of the experiments. HYL, JTD, and XZ performed part of the cell proliferation and Western blotting assay. YW established AMG510-acquired resistance cell lines. LX conducted part of animal experiments. LHM and YXW supervised the study. All authors proofread the manuscript and approved to submit the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Wl., Li, Hy., Wang, Y. et al. Targeting PI3Kα overcomes resistance to KRasG12C inhibitors mediated by activation of EGFR and/or IGF1R. Acta Pharmacol Sin 44, 1083–1094 (2023). https://doi.org/10.1038/s41401-022-01015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-01015-0