Abstract
Sevoflurane inhalation is prone to initiate cognitive deficits in infants. The early growth response-2 (Egr-2) gene is DNA-binding transcription factor, involving in cognitive function. In this study we explored the molecular mechanisms underlying the vulnerability to cognitive deficits after sevoflurane administration. Six-day-old (young) and 6-week-old (early adult) mice received anesthesia with 3% sevoflurane for 2 h daily for 3 days. We showed that multiple exposures of sevoflurane induced significant learning ability impairment in young but not early adult mice, assessed in Morris water maze test on postnatal days 65. The integrated differential expression analysis revealed distinct transcription responses of Egr family members in the hippocampus of the young and early adult mice after sevoflurane administration. Particularly, Egr2 was significantly upregulated after sevoflurane exposure only in young mice. Microinjection of Egr2 shRNA recombinant adeno-associated virus into the dentate gyrus alleviated sevoflurane-induced cognitive deficits, and abolished sevoflurane-induced dendritic spins loss and BDNF downregulation in young mice. On the contrary, microinjection of the Egr2 overexpression virus in the dentate gyrus aggravated learning ability impairment induced by sevoflurane in young mice but not early adult mice. Furthermore, we revealed that sevoflurane markedly upregulated the nuclear factors of activated T-cells NFATC1 and NFATC2 in young mice, which were involved in Egr2 regulation. In conclusion, Egr2 serves as a critical factor for age-dependent vulnerability to sevoflurane-induced cognitive deficits.
Similar content being viewed by others
Introduction
There is ample evidence to demonstrate a link between inhaled general anesthesia and postoperative learning ability impairment [1,2,3,4]. Observational studies have shown that exposure to anesthesia was associated with increased problems related to executive function, behavior, and reading in young children before the age of three [1,2,3]. Similarly, repeated exposure of infant rhesus monkeys to sevoflurane results in visual recognition memory impairment that emerges after the first year of life [4]. However, exposure to sevoflurane did not cause memory impairment in the adult brain [5,6,7,8]. Anesthesia-induced learning ability impairment and neuroinflammation was observed in young mice but not in adult mice [8]. These studies have shown age-related differences in susceptibility to learning ability impairment induced by exposure to general anesthesia. Sevoflurane, which is commonly used as a general anesthesia, triggers a series of pathophysiologic reactions, including the initiation of neuronal apoptosis [9], synaptogenesis impairment [10], and neuroinflammation [8, 11, 12] in young animals that result in cognitive deficits. These neurotoxic and learning ability impairment may also be mediated by neuronal receptors [13, 14], autophagy [15], neuroinflammation [16] and epigenetic mechanisms [17]. However, these various instances of molecular mechanisms failed to explain age-dependent learning ability impairments induced by sevoflurane.
The early growth response (EGR) genes consist of four members (Egr-1, -2, -3, and -4), which participate in synaptic and neuronal responses to external stimulation [18, 19]. Egr2 facilitates the robust and sustained maintenance of learning and memory by mediating synaptic plasticity [20]. Of particular interest, a previous study indicated that the performance of motor learning and object recognition memory was enhanced in Egr2-deficient mice [21]. These studies indicate that Egr2 is involved in synaptic plasticity and cognitive function. Moreover, previous studies reported that abnormal upregulation of Egr2 could be related to neurodevelopmental dysfunction after early-life stress [22,23,24]. Egr2 was also shown to be increased in young animals subjected to ischemic preconditioning [22]. De novo dominant mutations in Egr2 have been shown to be a cause of very early onset hereditary neuropathies [23]. Nevertheless, whether Egr2 contributes to the differences in cognition observed between young mice and early adult mice after general anesthesia remains to be investigated.
The focus of the present study is to explore the distinct vulnerability to cognitive deficits that occurs after sevoflurane administration. We use RNA-seq to screen the key gene regulation in learning ability impairment in neonatal mice but not in early adult mice. We determined that upregulation of Egr2 led to age-dependent decline in cognitive function after sevoflurane anesthesia. The present study provides the first evidence indicating that Egr2 is a critical factor in age-sensitive vulnerability to sevoflurane-induced learning ability impairment.
Materials and methods
Mice, anesthesia, and treatment
All experimental operations were in accordance with guidelines for laboratory animal care and safety from NIH and approved by the Animal Care and Use Committee of Zhejiang University (No. ZJU20160074) and Ethical Review Committee for laboratory animal welfare of Sir Run Run Shaw Hospital (No. SRRSH202102090). All animals were housed with free access to water and chow in appropriate environmental conditions (temperature: 22–25°C, humidity: 45%–50%, and 12 h light/dark cycle). Efforts were made to minimize the number of animals used in the studies.
The pups at 6–8 postnatal days were defined as neonatal mice. The mice, at 42–44 postnatal days treated with sevoflurane or not, were regarded as early adult mice. These neonatal mice after sevoflurane treatment or not grew up to postnatal 65 days were regarded as young mice according to previous studies [25, 26]. The male C57BL/6 mice (6 week) were purchased from Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, China). Neonatal mice of both sexes at postnatal day 6 were obtained from our own breeding. The neonatal mice were anesthetized on postnatal days 6, 7, and 8 with 3% sevoflurane delivered in humidified 20% oxygen carrier gas for 2 h daily for 3 days. The neonatal mice in the control group only received 20% oxygen at an identical flow rate in the same chambers. These neonatal mice after sevoflurane treatment or not grew up to postnatal 65 days were defined as young mice. The early adult mice were anesthetized on postnatal days 42, 43, and 44. The early adult mice in the control group only received 20% oxygen at an identical flow rate in the same chambers. The protocol was based on the previous study [27]. Skin temperature of the neonatal mice in the control and sevoflurane groups at different time point were detected (supplementary Table 1). Arterial carbon dioxide partial pressure (PaCO2), arterial oxygen pressure (PaO2), and power of hydrogen (pH) of young mice were evaluated by using a blood gas analyzer (Kent Scientific Corp., Torrington, CT, USA) (supplementary Table 2). There was no significant difference in pH, PaCO2 and PaO2 level between the groups of young mice. All mice were used in the further studies.
Morris water maze test
After the sevoflurane exposure and other experiments, the spatial memory was evaluated on postnatal days 60–65 by using the Morris Water Maze (MWM) test as previously described [28]. A circular black pool (diameter: 120 cm; depth: 21 cm) was filled with opaque water using white non-toxic ink to reach 1.0 cm above the platform surface (diameter, 10 cm), and the water temperature was kept at 22 °C. In the training phase, all animals received four training trials per day for a total of four days. The mice were placed into the pool at a special starting position and allowed to discover the hidden platform for 60 s. Mice were guided to the platform if they could not locate the platform within 1 min. The latency time (the time to reach the hidden platform) was recorded for assessing the spatial learning. After each trial, the mice were wiped dry and a heat lamp was used to faster temperature recovering before returning to home cages. In the testing phase, the platform was removed, and each mouse was allowed to swim freely in the pool for 2 min. The platform crossing times and the quadrant time were recorded for measuring memory function.
Viral packaging and stereotactic injection
The CMV-Egr2 shRNA and Egr2 overexpression adeno-associated virus were constructed and packaged by Vigene Biosciences (Shangdong, China). For Egr2 shRNA viral packaging, the shRNA sequence of mouse Egr2 (5'-GATCCGGGCAGGACAAAGCAATATTGTTCAAGAGACAATATTGCTTTGTCCTGCCCTTTTTTA-3') was synthesized and cloned into pAV-U6-GFP plasmid to produce pAV-U6-eGFP-Egr2 shRNA. Scramble adeno-associated virus was regard as the control virus. Viral particles were purified by iodixanol step-gradient ultracentrifugation. The genomic titer was 4.72 × 1013 TU/mL determined by quantitative PCR. For Egr2 overexpression, the coding sequence of mouse Egr2 was synthesized and cloned into PAG-CAG-eGFP plasmid to produce PAG-CAG-Egr2-P2A-eGFP. The eGFP plasmid virus was produced as control virus. Viral particles were purified by iodixanol step-gradient ultracentrifugation. The genomic titer was 4.68 × 1013 TU/mL determined by quantitative PCR.
For viral injection, mice were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg) by intraperitoneal injection and placed in a stereotactic frame. Purified and concentrated adenovirus was injected bilaterally into the dentate gyrus (100 nL, coordinates from bregma, − 1.5 mm anterior/posterior, − 2.07 mm medial/lateral, −1.8 mm dorsal/ventral) through glass micropipettes at a slow rate (10 nL/min). In the animal experiments, the young mice received virus injection (100 nL) on postnatal days 9 after being anesthetized with sevoflurane, and the adult mice (100 nL) received virus injection on postnatal days 45. The Morris water maze tests of all mice were performed on postnatal days 65. The injection sites were examined at the end of all the behavioral tests and only data from animals with correct injections were collected. Sections through the dentate gyrus were directly examined under a fluorescence microscope.
Tissue harvest
The brain tissues of young and early adult mice were harvested on postnatal days 65 after Morris Water Maze tests. The brain from animals were anesthetized with 2% pentobarbital sodium (40 mg/kg, i.p.). Then, the right atrium was incised and transcardiac perfusion was performed with heparinized 0.9% saline followed by 4% formaldehyde. The brain tissue was extracted and rinsed using 0.9% sodium chloride at 4 °C. The brain was stripped and fixed in 4% formaldehyde for overnight, in 15% sucrose in 0.1 M phosphate buffers (pH 7.4, 4 °C) for 24 h, and the in 30% sucrose in 0.1 M phosphate buffers (pH 7.4, 4 °C) for 24–48 h. The specimens were stored in a −80 °C freezer.
Next-generation sequencing
For the RNA-Seq analysis, the hippocampal tissues were obtained from young and adult mice treated with sevoflurane both at 65 postnatal days. Total RNA was extracted from different groups using RNAiso Plus Reagent (TaKaRa, Japan), and purified using an RNasey Mini Kit (QIAGEN) based on the manufacturer’s protocol. NanoDrop spectrophotometry (Thermo Scientific, Wilmington, USA) was used to determine the RNA concentration, and the integrity was confirmed through electrophoresis. Subsequently, the cDNA synthesis and antisense RNA (aRNA) amplification was performed using Amino Allyl MessageAmp II aRNA Amplification Kit (Ambion, USA). The total RNA was stored at −80 °C for future use.
A total of 1.5 μg of RNA was used as the input material. The clustering of the index-coded samples was performed using a TruSeq PE Cluster Kit v3-cBot-HS (Illumina) based on the manufacturer’s instructions. The library was sequenced using an Illumina HiSeq platform, and paired-end reads were generated followed by cluster generation. Thereafter, raw reads in the fastq format were processed by using in-house Perl scripts. Low-quality data were discarded using Trim Galore. The GC-content and sequence duplication level of the clean reads were calculated, and the clean reads were assembled with Trinity Software via the default parameters. Then, the RNA-Seq data files were deposited in the NCBI Sequence Read Archive (SRA) database (SRA accession: PRJNA781082).
The differentially expressed genes (DEGs) acquired from the RNA-seq-based expression profiling were analyzed through iDEP (integrated Differential Expression and Pathway analysis) online tools. Currently, iDEP seamlessly connects 63 R/Bioconductor packages, 2 web services, and comprehensive annotation and pathway databases for 220 plant and animal species [29]. Briefly, the expression matrix was filtered only for transcripts with >5 reads per kilobase of transcript per million mapped reads (RPKM). The pairwise comparison was employed using the DESeq2 package with a threshold of false discovery rate FDR < 0.05 and |log2FC| > 1. Additionally, hierarchical clustering of genes was done using Pearson correlation distance and average linkage. Principal component analysis for the hippocampus tissues was done using Log2(TPM+1) values for genes determined to be differentially expressed by one-way ANOVA. Genes specifically upregulated for either cytokine were identified by selecting those that had at least a |log2FC| > 1 and a false discovery rate FDR < 0.05 in one condition compared with all of the other three.
Golgi-Cox staining
The morphology of neuronal dendrites and dendritic spines was investigated in the dentate gyrus by using the Hito Golgi-Cox OptimStainTM PreKit (Hitobiotec Corp. Kingsport, TN, USA). The brain tissues were obtained after sacrifice and rinsed with Milli Q water. The equal volumes of Solutions A and B were used to impregnate the brain tissues, and the impregnation solution was replaced the following day and stored in darkness (Room temperature, 2 weeks). Then, the brain tissues were transferred to Solution C, which was replaced the following day. The brains were stored at 4 °C for 72 h in the dark. The brain sections (100 μm thickness) were generated using a cryotome with the chamber temperature set at −19 °C. Each section was mounted on gelatin-coated microscope slides using Solution C. The excess solution on slide was removed using a Pasteur pipette and absorbed with filter papers, then the sections were allowed to dry naturally at room temperature for 3 days. The dried brain sections were processed according to the manufacturer’s instructions. Thereafter, the dendrites of dentate gyrus sub region in the hippocampus were observed by using an Olympus BX61 fluorescence microscope (Olympus, Japan).
Western blotting
The hippocampal tissues among different groups were homogenized using RIPA buffer (Beyotime, P0013B) with 1 × protease inhibitor cocktail (Beyotime, P1010). The supernatant was collected by centrifugation (12,000 × g, 20 min), and the protein concentration was measured through a bicinchoninic acid protein assay kit (Beyotime, P0012S). An aliquot of 50 µg protein was separated via SDS-PAGE and transferred to a nitrocellulose membrane, then blocked with 5% nonfat milk in phosphate-buffered saline (PBS, pH 7.4). The membranes were incubated with primary antibodies against Egr2 (1:1, 000; ABclonal, A15053), Egr1 (1:500; ABclonal, A2722), Egr4 (1:1000; ABclonal, A16369), Arc (1:500; ABclonal, A9177), BDNF (1:1, 000; abcam, ab108319), NFATC1 (1:1,000; ABclonal, A1539), NFATC2 (1:1000; ABclonal, A3107), NFATC3 (1:1000; ABclonal, A6666), actin (1:5, 000; ABclonal, AC026) at 4 °C overnight. Blots were incubated in horseradish peroxidase-conjugated secondary antibodies against rabbit IgG (1:5, 000, CST, 7071 and 7072) for 2 h at room temperature, then subjected to chemiluminescent detection using the SuperSignal West Pico Substrate (34077, Pierce) and exposed to film. Digital images were quantified using densitometric measurements obtained using Quantity One software (Bio-Rad).
Immunofluorescent staining
Serial coronal slices (20 μm thick) of hippocampus including CA1, CA3 and DG regions were made by using a rotary microtome (Leica, Germany). The sections were blocked using 10% bovine serum albumin at room temperature for 1 h. The sections were incubated with antibody diluent containing goat antibodies against Egr2 (1:200; NOVUS biologicals, OTI1B12), Iba-1 (1:100; abcam, ab178846) and NeuN (1:1000; abcam, ab104224) overnight at 4 °C. Then sections were rinsed with PBS (3 × 10 min) followed by incubation with Alexa Fluor™ 488 goat anti-mouse antibody and Alexa Fluor™ 594 goat anti-rabbit antibody for 1 h at room temperature. After rinsing with PBS (6 × 5 min), fluorescence signals were visualized under an epifluorescence microscope. Images were captured with the assistance of Image-Pro Plus 5.0 software, and all the parameters used were kept consistent during capturing.
Statistical analysis
We present the data from biochemical studies, escape latency, platform crossing times and quadrant time of Morris water maze as means ± SD; we defined young mice as the reference group and set values of variables in the reference group mice without anesthesia for comparison in experiments comparing young and adult mice. All data were quantified and expressed as ratio (Western blot) or real numbers (number of dendritic spines) of the reference group or baseline group. Interaction between time and group factors was determined by using two-way ANOVA with repeated measurements to analyze the difference in learning curves (escape latency) between mice in the baseline and sevoflurane anesthesia groups in the Morris water maze test. The ordinary one-way ANOVA and Tukey’s multiple comparisons test were used to determine the difference between the baseline and sevoflurane anesthesia groups in terms of platform crossing times and quadrant time. The ordinary one-way ANOVA and Tukey’s multiple comparisons test were used to determine the difference in FPKM between the baseline and sevoflurane anesthesia groups. Two-way ANOVA and Student’s t test with Bonferroni correction were used to determine the effect of age (postnatal day 6 vs. postnatal week 6), treatment (baseline vs. sevoflurane anesthesia), and the interaction of age and treatment in terms of total amounts of Arc, Egr1, Egr2, Egr4, BDNF, NFATC1, NFATC2 and NFATC3. Two-way ANOVA and the Tukey’s multiple comparisons test were used to determine the difference in the effect of Egr2 shRNA and treatment (baseline vs sevoflurane anesthesia) in terms of the dendritic numbers. P < 0.05 was considered statistically significant, and significance testing was two-tailed. Statistical analysis was conducted using GraphPad Prism software (version 8.0) and SPSS statistic software (version 21.0).
Results
Sevoflurane induces learning ability impairment in young mice but not in early adult mice
We firstly compared the spatial memory of 6-day-old mice and 6-week-old mice after exposure to sevoflurane. For neonatal mice, exposure to sevoflurane (3% + 20% oxygen) occurred on postnatal day 6–8 (2 h daily). For early adult mice, sevoflurane was administered at postnatal days 42–44. The mice in the control group only received 20% oxygen. The impact of sevoflurane on cognitive function across all male mice was measured using the Morris water maze on postnatal days 60–65 (Fig. 1a). In young mice, the mice exposed to sevoflurane had longer escape latency when locating the maze exist as compared to the control mice (P < 0.0001). Mice that received sevoflurane also demonstrated lower platform crossing times (Y-Ctrl: 4.625 (1.061) times (mean (SD)) vs Y-SEV: 2.375 (0.916) times, P = 0.0049) and shorter quadrant time (E-Ctrl: 33.72 (3.669) % vs E-SEV: 27.20 (4.949) %, P = 0.0094) compared to control mice. In early adult mice, the escape latency, platform crossing times, and quadrant time were not significantly altered by sevoflurane treatment (Fig. 1b–e). These results suggest that sevoflurane anesthesia induces learning ability impairment in young mice but not in early adult mice, which demonstrates that age-sensitive vulnerability to learning ability impairment is associated with the use of sevoflurane.
a Experimental design. The neonatal mice received 3% sevoflurane for 2 h daily on postnatal days 6, 7, and 8. The early adult male mice at 6 weeks old were anesthetized in the same way. The spatial memory of all the male mice were be measured by Morris water maze on postnatal days 60–65. b Representative path patterns of Morris water maze in the test day of each group. c–e Escape latency (c), platform crossings (d) and quadrant time (e) of Morris Water maze test after sevoflurane treatment (n = 8 mice/group). The data were presented as means ± SD. Two-way ANOVA was used for (c–e). **P < 0.01, ***P < 0.001.
Sevoflurane upregulates Egr2 expression in young mice but not in early adult mice
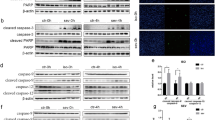
To characterize a genome-wide expression profile corresponding to sevoflurane use, we employed RNA-seq high-throughput sequencing to determine gene expression changes in all mice after exposure to sevoflurane at 65 postnatal days. We found that differentially expressed genes (DEGs) mainly comprised 57 upregulated and 16 downregulated genes in young mice, and 21 upregulated genes and 37 downregulated genes in early adult mice (Fig. 2a). In the RNA-sequencing data, the FPKM level of Egr1 (Y-Ctrl: 21.35 (3.305) vs Y-SEV:50.60 (4.428), P < 0.0001; E-Ctrl: 46.14 (3.068) vs E-SEV: 56.98 (2.501), P = 0.019) and Arc (Y-Ctrl:27.53 (4.013) vs Y-SEV: 81.57 (14.66), P = 0.003; E-Ctrl: 69.55 (15.54) vs E-SEV: 91.91 (11.68), P = 0.038) were upregulated both in young mice and in early adult mice after sevoflurane treatment. The FPKM level of Egr2 (Y-Ctrl: 0.3719 (0.117) vs Y-SEV:2.390 (0.404), P = 0.0025) and Egr4 (Y-Ctrl:11.94 (1.826) vs Y-SEV:30.77 (2.256), P < 0.0001) were upregulated in young mice but not in early adult mice (Fig. 2b). To further evaluate the protein expression of Arc, Egr1, Egr2, and Egr4, we compared the amounts of these proteins between young and early adult mice at control baseline levels and after exposure to sevoflurane. Western blot analysis revealed that the expression of Arc (Y-Ctrl: 1.024 (0.046) vs Y-SEV:1.546 (0.129), P = 0.0001; E-Ctrl: 1.086 (0.099) vs E-SEV: 1.410 (0.049), P = 0.0215) and Egr1 (Y-Ctrl: 0.999 (0.103) vs Y-SEV:2.948 (0.175), P < 0.0001; E-Ctrl: 0.882 (0.1125) vs E-SEV: 3.068 (0.233), P < 0.0001) increased in young mice and early adult mice after exposure to sevoflurane as compared with baseline non-anesthetized mice (Fig. 2c, d). The expression of Egr4 (Y-Ctrl:1.161 (0.060) vs Y-SEV: 1.842 (0.321), P < 0.0001) increased after sevoflurane treatment in early adult mice but not in young mice, which was inconsistent with the results of RNA-sequencing and MWM data. These results suggest that sevoflurane may affect the expression of Egr4 through a transcriptional mechanism. Interestingly, the expression of Egr2 (Y-Ctrl: 1.031 (0.059) vs Y-SEV: 2.174 (0.068), P < 0.0001) increased after sevoflurane treatment in young mice but not in early adult mice, which was supported by the Morris water maze results (Fig. 2c, d). These data indicate that sevoflurane exerted a significant influence on the transcriptome and Egr2 could be a critical gene in the occurrence of age-sensitive vulnerability to sevoflurane-induced learning ability impairment.
a Hierarchical clustering and select representative genes between the young mice and early adult mice after sevoflurane treatment. b Normalized expression (FPKM) of select representative genes in the young mice and early adult mice after sevoflurane treatment. (n = 3 mice/group). FPKM Fragments Per Kilobase of exon model per Million mapped fragments. c Amounts of Arc, Egr1, Egr2, and Egr4 in the dentate gyrus of the young mice and early adult mice in baseline and sevoflurane anesthesia groups. d Summary of Arc, Egr1, Egr2, and Egr4. (n = 5 mice/group). The data were presented as means ± SD. Two-way ANOVA was used. *P < 0.05, **P < 0.01, ***P < 0.001.
The RNA-seq data demonstrated that sevoflurane treatment resulted in an upregulation in 57 genes and downregulation in 16 genes in young mice. The differentially expressed genes were frequently involved in transcriptional regulator activity, synapse formation, developmental processes, and so on according to GO function analysis (Supplementary Fig. 1).
We measured the expression of Egr2 by immunofluorescent staining. The brain sectioning images revealed that sevoflurane increased the expression of Egr2 mainly in the dentate gyrus (Supplementary Fig. 2a) (F = 5.938; P < 0.0001). Extending this analysis to Egr2-specific cell types, we observed Egr2 colocalization with NeuN (neuronal marker) but not Iba-1 (microglia marker) (Supplementary Fig. 2b). These data suggest that Egr2 is mainly expressed in neurons of the dentate gyrus.
Egr2 is required for sevoflurane-induced cognitive deficits in young mice
To determine the effect of Egr2 on sevoflurane-induced cognitive function in young mice, we transfected an Egr2 shRNA recombinant adeno-associated virus (AAV) into the dentate gyrus of mice by intracerebroventricular injection. The mice received anesthesia with 3% sevoflurane 2 h daily at P6-P8 and were injected with Egr2 shRNA viruses at P9. The mice were sacrificed at P65 after they completed a Morris water maze (Fig. 3a). The injection sites were post-validated at the end of the Morris water maze (Fig. 3b). We utilized Western blots to examine the efficiency of adeno-associated virus infection. These results showed that Egr2 shRNA abolished expression of Egr2 protein in mice (Fig. 3c) (scramble-Ctrl:1.033 (0.056) vs Egr2 shRNA-Ctrl:0.385 (0.056), P = 0.028). In young mice that received scramble virus, sevoflurane induced learning ability impairment, as measured using a Morris water maze to test for escape latency (P < 0.0001, scramble-Ctrl vs scramble-SEV), platform crossing time (scramble-Ctrl: 6.833 (1.169) times vs 2.833 (1.169) times, P < 0.0001) and quadrant time (scramble-Ctrl: 34.26 (2.165) % vs scramble-SEV: 25.03 (6.071) %, P = 0.0027), as compared with baseline non-anesthetized mice. In the young mice with sevoflurane and Egr2 shRNA treatment, escape latency was significantly shortened, (P = 0.0002, scramble-SEV vs Egr2 shRNA-SEV) platform crossing times was increased, (scramble-SEV:2.833 (1.169) times vs Egr2 shRNA-SEV: 5.833 times (1.169) P = 0.001,) and quadrant time was longer (scramble-SEV:25.03 (6.071) % vs Egr2 shRNA-SEV:31.67 (3.558) % P = 0.0347) when compared to sevoflurane mice (Fig. 3e–h). These data indicate that Egr2 shRNA alleviates sevoflurane-induced cognitive deficits in young mice.
a Experimental design. The neonatal mice were given 3% sevoflurane for 2 h daily on postnatal days 6, 7, and 8, and injected Egr2 shRNA virus on postnatal days 9 in the following of Morris water maze on postnatal days 60–65. b Representative images of the expression of Egr2 shRNA in the dentate gyrus of a naïve mouse. Scale bars, 1 mm. c Amounts of Egr2 in the dentate gyrus of the mice on postnatal days 65 after sevoflurane treatment and Egr2 shRNA virus injection. d Summary of Egr2. (n = 3 mice/group). e Representative path patterns of Morris water maze in the test day in each group. f–h Escape latency (f), platform crossings (g) and quadrant time (h) of Morris Water maze test after sevoflurane treatment and Egr2 shRNA virus injection (n = 6 mice/group). The data were presented as means ± SD. Two-way ANOVA was used for (d, f, g, h). *P < 0.05, **P < 0.01, ***P < 0.001.
Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron’s cell body [25]. The number of dendritic spines, as well as spine morphology, has been regarded as a good way to measure the activity of neurons [26]. We determined the number of dendritic spines within the dentate gyrus after sevoflurane and Egr2 shRNA treatment by Golgi staining. These results showed that sevoflurane decreased the number of dendritic spines in the dentate gyrus of mice injected compared with vehicle virus (scramble-Ctrl: 10.53 (2.649) vs scramble-SEV: 5.33 (2.537), P < 0.0001). However, Egr2 shRNA rescued the reduced number of dendritic spines induced by exposure to sevoflurane (Fig. 4a, b) (scramble-SEV: 5.333 (2.537) vs Egr2 shRNA-SEV: 7.867 (2.980), P = 0.0023). Brain-derived neurotrophic factor (BDNF) has been shown to play a role in neuroplasticity, which allows nerve cells in the brain to compensate for injury, new situations, or changes in their environment [27]. In our study, BDNF expression was found to decrease in mice after sevoflurane treatment (scramble-Ctrl: 1.023 (0.048) vs scramble-SEV: 0.765 (0.151), P = 0.0052). However, Egr2 shRNA was able to alleviate decreased expression of BDNF associated with sevoflurane treatment (Fig. 4c, d) (scramble-SEV: 0.765 (0.151) vs Egr2 shRNA-SEV: 0.961 (0.082), P = 0.0047). Collectively, Egr2 shRNA rescued dendritic spines loss and decreased BDNF expression induced by sevoflurane in young mice.
a Representative images of Golgi staining in the dentate gyrus of the mice on postnatal days 65 after sevoflurane treatment and Egr2 shRNA virus injection. Scale bars, 20 μm. b Amounts of Egr2 and BDNF in the dentate gyrus of the mice on postnatal days 65 after sevoflurane treatment and Egr2 shRNA virus injection. c Analysis of the number of dendritic spines at the unit of 20 μm. More than 30 images were counted for each group. d Relative protein level of Egr2 and BDNF. (n = 3 mice/group) (e) Experimental design. The neonatal mice were given 3% sevoflurane for 2 h daily on postnatal days 6, 7, and 8, and injected BDNF virus on postnatal days 9. f Representative images of Golgi staining in the dentate gyrus of the mice on postnatal days 65 after sevoflurane treatment and BDNF virus injection. Scale bars, 20 μm. g Analysis of the number of dendritic spines at the unit of 20 μm. More than 20 images were counted for each group. The data were presented as means ± SD. Two-way ANOVA was used for (b, d). *P < 0.05, **P < 0.01, ***P < 0.001.
We also explored the effect of upregulating BDNF in young mice after sevoflurane treatment. Neonatal mice were treated with sevoflurane on postnatal days 6, 7, and 8, and then injected with a BDNF overexpression adeno-associated virus into their dentate gyrus at postnatal day 9. Golgi staining was performed on P65. As shown in Fig. 4e–g, upregulation of BDNF in the hippocampus reversed sevoflurane-induced spine loss in young mice (scramble-SEV: 6.900 (2.426) vs BDNF-OE: 9.582 (2.885), P = 0.0224). Altogether, these data indicate that Egr2 is required for sevoflurane-induced cognitive deficits in young mice.
Egr2 overexpression did not induce cognitive deficits in early adult mice
To further verify that Egr2 was sufficient for cognitive function, young mice were injected with a virus overexpressing Egr2 into their dentate gyrus on postnatal day 9 following sevoflurane anesthesia for 3 days. Early adult mice were injected with the Egr2 overexpression virus into their dentate gyrus on postnatal day 45 following sevoflurane treatment from P42 to P44. Morris water maze tests were performed on all male mice at P60-P65 (Fig. 5a). The memory behavior tests of young mice revealed that injection with the Egr2 overexpression virus increased escape latency (P < 0.0001, GFP vs Egr2 OE) and decreased platform crossing (GFP:8.833 (2.317) times vs Egr2 OE:4.500 (1.049) times, P = 0.0008) and quadrant time (GFP: 45.26 (32.34) % vs Egr2 OE 32.34 (7.911) %, P = 0.0214) as compared to mice that were administered the vehicle virus (Fig. 5b–e). Moreover, after sevoflurane treatment, treatment with the Egr2 overexpression virus was able to decrease platform crossings times (GFP-SEV: 5.500 (1.643) times vs Egr2 OE-SEV: 2.500 (1.049) times, P = 0.0196) and shorten quadrant time (GFP-SEV: 31.78 (6.672) % vs Egr2 OE-SEV: 19.67 (3.034) %, P = 0.033) in young mice compared to mice in the control virus group. These results suggest that the Egr2 overexpression virus aggravates learning ability impairment after sevoflurane treatment. In early adult mice, treatment with the Egr2 overexpression virus had no significant influence on escape latency, platform crossings, and quadrant time with or without sevoflurane treatment (Fig. 5b, f–h). These data indicate that upregulated Egr2 expression was sufficient in causing cognitive deficits induced by sevoflurane treatment in young mice, and Egr2 is a critical gene in the mediation of age-dependent learning ability impairment induced by sevoflurane.
a Experimental design. The neonatal mice were given 3% sevoflurane for 2 h daily on postnatal days 6, 7, and 8, and injected Egr2 overexpression virus on postnatal days 9 in the following of Morris water maze on postnatal days 60–65. The early adult mice were given 3% sevoflurane for 2 h daily on postnatal days 42–44, and injected Egr2 overexpression virus on postnatal days 45 in the following of Morris water maze on postnatal days 60–65. b Representative path patterns of Morris water maze in the test day in each group of young mice and early adult mice. c–e Escape latency (c), platform crossings (d) and quadrant time (e) of Morris Water maze test after sevoflurane treatment in young mice (n = 6 mice/group). f–h Escape latency (f), platform crossings (g) and quadrant time (h) of Morris Water maze test after sevoflurane treatment in early adult mice (n = 6 mice/group). The data were presented as means ± SD. Two-way ANOVA was used for (c–h). *P < 0.05, **P < 0.01, ***P < 0.001.
Based on the effects of the Egr2 overexpression virus on cognitive function after sevoflurane treatment in young mice, we next determined the number of dendritic spines in the dentate gyrus using a Golgi staining assay, and we found that the Egr2 overexpression virus decreased the number of dendritic spines in the dentate gyrus (Supplementary Fig. 3a, b) (GFP: 9.633 (2.918) vs Egr2 OE 7.000 (2.197), P = 0.005). We also found that expression of BDNF dropped significantly in mice administered the Egr2 overexpression virus (Supplementary Fig. 3c–d) (GFP:1.033 (0.012) vs Egr2 OE:1.812 (1.692), P = 0.0323).
Sevoflurane induces NFAT activation, which regulates Egr2 expression
We next explored how sevoflurane treatment regulates expression of Egr2. A previous study indicated that Egr2 was transcriptionally regulated by NFAT [28]. In a protein-protein interaction network (Fig. 6a), we found there were strong connections between Egr2 and BDNF, and Egr2 and NFAT. The network suggested that sevoflurane treatment upregulated NFAT, which is involved in Egr2 regulation (Fig. 6a). The nuclear factor of activated T-cells (NFAT) is a family of transcription factors comprising four calcium-regulated members: NFATc1, NFATc2, NFATc3, and NFATc4. NFATs translocate from the cytosol to the nucleus and regulate their target genes, which are involved in axon growth, synaptic plasticity, and neuronal survival in the nervous system [29]. Western blotting revealed that sevoflurane upregulated expression of NFATC1 (Y-Ctrl:1.033 (0.090) vs Y-SEV:2.939 (0.161), P < 0.0001) and NFATC2 (Y-Ctrl:1.045 (0.057) vs Y-SEV:2.039 (0.113), P < 0.0001) in young mice but not in early adult mice, and increased expression of NFATC3 in both young mice and early adult mice (Fig. 6b, c) (Y-Ctrl:1.000 (0.098) vs Y-SEV:2.944 (0.478), P < 0.0001; E-Ctrl:1.042 (0.082) vs E-SEV:2.020 (0.0091), P < 0.0001). These data suggest that sevoflurane treatment can regulate Egr2 expression in young mice through the activation of NFATC1 and NFATC2.
a Protein-protein interaction network construction for differential expression genes (DREs) in young mice after sevoflurane treatment. b Amounts of NFATC1, NFATC2 and NFATC3 in the dentate gyrus of the young mice and early adult mice after sevoflurane treatment on postnatal days 60–65. c Summary of NFATC1, NFATC2 and NFATC3. (n = 5 mice/group). The data were presented as means ± SD. Two-way ANOVA was used for (b). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
There is clear evidence demonstrating a link between early-life anesthesia exposure and increased risk of subsequent sustained neurobehavioral deficits [12]. However, the underlying mechanisms driving age-dependent learning ability impairment induced by sevoflurane remain unknown. In this study, we investigated the discrepant vulnerability to cognitive deficits induced by sevoflurane administration using transcriptomics. We found that sevoflurane treatment resulted in an age-specific upregulation of Egr2 in infant mice that was associated with cognitive deficits. Moreover, upregulated Egr2 was required for the cognition defects observed in adulthood. Remarkably, ectopic expression of Egr2 in the hippocampus of young, but not early adult, mice was sufficient to reinforce sevoflurane-induced spatial learning impairment. For the first time, these data demonstrated that Egr2 serves as a critical factor for the occurrence of age-sensitive vulnerability to learning ability impairment induced by sevoflurane.
The present study found that upregulated Egr2 led to age-dependent learning ability impairment induced by sevoflurane. We profiled the transcriptome of young mice and early adult mice after sevoflurane treatment. RNA-seq results confirmed that Arc, whose expression is relative to neuronal synaptic plasticity [30], was upregulated after sevoflurane treatment in both young mice and early adult mice. This finding is supported by a previous study that identified sevoflurane significantly increased Arc protein expression in the hippocampus [31]. A previous study also explored the transcriptomic profile in the hippocampus of young mice after exposure to sevoflurane [32]. However, the effect of sevoflurane on key proteins in an age-dependent manner associated with cognitive deficits had not been explored in that study. Interestingly, we found that the RNA level of Egr2 according to the RNA-seq data and altered expression of Egr2 as determined by Western blotting were all consistent with the impact of sevoflurane based on Morris water maze performance (Fig. 2). These results suggest a critical role for Egr2 in age-dependent cognitive dysfunction induced by sevoflurane exposure. Egr2 has also been shown to play an important role in the maintenance of learning and memory by mediating synaptic plasticity [20, 33, 34]. Egr2-deficient mice had improved performance in motor learning and recognition memory [21]. Here, we utilized Egr2 shRNA viruses to alter the expression of Egr2 in the hippocampus. The Egr2-silencing virus alleviated sevoflurane-induced spatial memory impairment in young mice (Fig. 3). Our current findings further emphasize that Egr2 is required for age-sensitive vulnerability to learning ability impairment induced by sevoflurane. Moreover, overexpression of Egr2 was sufficient for sevoflurane-induced spatial memory impairment in young mice, but not in early adult mice (Fig. 5). Memory deficits induced by early-life stress have been associated with Egr2 malfunction and aberrant activation of its downstream signaling pathways [23, 24, 35]. These studies further support the idea that Egr2, a transcription factor, is sensitive to sevoflurane at early stages of life. A recent study revealed lower concentrations of ATP in neonatal mice brains compared with adult mice was a contributor to age-dependent learning ability impairment after sevoflurane treatment [36]. In acute myocardial infarction, silencing of Egr2 reduced cardiomyocyte apoptosis and increased ATP content [37]. These findings may explain the effect of Egr2 on age-dependent learning ability impairment partly by regulating the concentrations of ATP in young mice brain. Future studies are needed to determine the underlying interactions between Egr2 and ATP concentrations.
The Egr family of genes, including Egr1, Egr2, Egr3, and Egr4, regulates cognitive function. Egr1 is promptly expressed after induction of long-term potentiation (LTP), which mediates cognitive function in aged animals but not in young animals [38,39,40]. In our RNA-sequencing data, Egr1 was upregulated after sevoflurane treatment in both young mice and early adult mice. It appears that Egr1 was not a key gene in the age-dependent learning ability impairment induced by sevoflurane treatment. Egr3 impairment has been associated with various neurodevelopmental dysfunctions [41,42,43]. However, sevoflurane had no effect on the transcript levels of Egr3 in the present study. A previous study suggests Egr4 expression plays a factor in schizophrenia with a reduction of Egr4 gene expression having been observed in male schizophrenia patients [44]. And the results in our research clued that sevoflurane may decrease Egr4 production in young mice through a posttranscriptional mechanism. Egr4 has Ser230 at a phosphorylation site in Mus musculus and T534 and T536 in Homo sapiens in the database of PhosphoSitePlus, v6.6.0.2. Egr4 had no glycosylation site in either Mus musculus nor in Homo sapiens according to O-GlcNAC Database v1.2. As for SUMOylation site (sumosp.biocuckoo.org) and we did not find any information about Egr4 in database. It’s valuable to further research the effect of Egr4 on sevoflurane-induced impairment of cognition through the phosphorylation site Ser230 with available methods.
As opposed to Egr1 and Egr3, Egr2 acts as an inhibitory constraint for cognitive function [45]. Egr2 mediates learning ability impairment via regulating the expression of BDNF [46]. The Egr2 shRNA virus rescued the decreased level of BDNF induced by sevoflurane treatment (Fig. 4). The expression of BDNF in the brain may facilitate protective and regenerative effects following brain injury [47]. Dendritic spine numbers have been shown to be affected by BDNF expression [48, 49], which further explains the effect of Egr2 on dendritic spines after sevoflurane treatment in the hippocampus. Induction of BDNF regulated by Egr2 contributed to age-dependent learning ability impairment induced by sevoflurane.
Nuclear factor of activated T-cells (NFAT) is a family of transcription factors comprising four members: NFATc1, NFATc2, NFATc3, and NFATc4. A high level of Egr2 is also induced following T cell activation, possibly via NFAT-mediated signaling [50, 51]. NFATs regulate a series of genes involved in axon growth, synaptic plasticity, and neuronal survival [29, 52]. General anesthesia-induced learning ability impairment has been shown to involve the activation of NFAT [53]. Here, we utilized NMDC101 (N-(4-chloro-2-fluorophenyl)-2-hydroxybenzamide) to downregulate the activation of NFATC1. In our present study, neonatal mice received 3% sevoflurane for 2 h daily on postnatal days 6, 7, and 8, and were then injected with NDMC101 (62.5 mg/kg, i.p.) on postnatal days 6–9. Golgi staining and Western blot assay were then performed. As shown in the supplementary figures, NDMC101 downregulated expression of NFATC1. However, NDMC101 did not block sevoflurane upregulation of Egr2, downregulation of BDNF, or spine loss in the hippocampus (Supplementary Fig. 4). Sevoflurane could regulate Egr2 expression in young mice by the activation of NFATC1 and NFATC2 (Fig. 6). Sevoflurane may still activate the expression of NFATC2 when NDMC101 administration. However, there is no current research about the effect of NDMC101 on NFATC2, and no commercially available NFATC2 inhibitors. It may help us to further explore the action of NFATC2 in young mice after sevoflurane treatment if we were to establish a special NFATC2 downregulation virus. Above all, our results indicate that sevoflurane could regulate Egr2 expression in young mice by the activation of both NFATC1 and NFATC2.
In clinical practice, there are no biochemical indicators for evaluating learning ability impairment in children after general anesthesia exposure. It is also not known whether increasing levels of Egr2 could be detected in serum of children. The current study provides a potential strategy to predict the risk of cognition impairment by sevoflurane treatment in children. In addition, BDNF regulation by Egr2 contributes to age-dependent learning ability impairment induced by sevoflurane, and agonists of BDNF could be a promising drug candidate for the treatment of children presenting with learning ability impairment after general anesthesia exposure.
There have been clinical trials assessing volatile anesthetic effects on postoperative cognition [54]. However, several studies have reported that aged mice suffered postoperative cognitive dysfunction (POCD) after general anesthesia without any surgery [55, 56]. As for neonatal mice, POCD has been similarly reported when mice receive general anesthesia without any surgery [16, 36, 57,58,59]. Nevertheless, the effects of anesthesia without surgery may be different from how POCD presents in clinical practice. In the present study, we only detected the learning ability of young and early adult mice at P60-P65 days. Several reports have suggested that mice continue to mature until the end of the third postnatal month [60, 61]. The water maze test, or other behavior tests, would be given at the different age period before sevoflurane exposure and tests were performed in the young and early adult.
Taken together, the current study explored the underlying mechanisms of age-related susceptibility to learning ability impairment induced by sevoflurane exposure. For the first time, we have conclusively shown that Egr2 is a critical gene in the regulation of age-dependent learning ability impairment after sevoflurane treatment. The present study provides a novel indicator to assist in the prediction of cognitive impairment in clinical practice.
References
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD. et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61.
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the mayo anesthesia safety in kids (MASK) study. Anesthesiology. 2018;129:89–105.
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82.
Alvarado MC, Murphy KL, Baxter MG. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119:517–23.
Kokubun H, Jin H, Komita M, Aoe T. Conflicting actions of inhalational anesthetics, neurotoxicity and neuroprotection, mediated by the unfolded protein response. Int J Mol Sci. 2020;21:450.
Liang P, Li F, Liu J, Liao D, Huang H, Zhou C. Sevoflurane activates hippocampal CA3 kainate receptors (Gluk2) to induce hyperactivity during induction and recovery in a mouse model. Br J Anaesth. 2017;119:1047–54.
Liu H, Chen B, Guo B, Deng X, Wang B, Dou X. Postconditioning with sevoflurane or propofol alleviates lipopolysaccharide-induced neuroinflammation but exerts dissimilar effects on the NR2B subunit and cognition. Mol Neurobiol. 2021;58:4251–67.
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG. et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15.
Xie SN, Ye H, Li JF, AnLX. Sevoflurane neurotoxicity in neonatal rats is related to an increase in the GABAA R alpha1/GABAA R alpha2 ratio. J Neurosci Res. 2017;95:2367–75.
Amrock LG, Starner ML, Murphy KL, Baxter MG. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122:87–95.
Ji MH, Qiu LL, Yang JJ, Zhang H, Sun XR, Zhu SH, et al. Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology. 2015;46:155–64.
Alam A, Suen KC, Hana Z, Sanders RD, Maze M, Ma D. Neuroprotection and neurotoxicity in the developing brain: an update on the effects of dexmedetomidine and xenon. Neurotoxicol Teratol. 2017;60:102–16.
Yu X, Zhang F, Shi J. Neonatal exposure to sevoflurane caused cognitive deficits by dysregulating SK2 channels and GluA2-lacking AMPA receptors in juvenile rat hippocampus. Neuropharmacology. 2018;141:66–75.
Liu Y, Yang H, Fu Y, Pan Z, Qiu F, Xu Y, et al. TRPV1 antagonist prevents neonatal sevoflurane-induced synaptic abnormality and cognitive impairment in mice through regulating the Src/cofilin signaling pathway. Front Cell Dev Biol. 2021;9:684516.
Wang X, Dong Y, Zhang Y, Li T, Xie Z. Sevoflurane induces cognitive impairment in young mice via autophagy. PLoS One. 2019;14:e0216372.
Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou Z, et al. Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-kappaB pathway in neonatal mice. J Nutr Biochem. 2021;90:108579.
Zhu Y, Wang Y, Yao R, Hao T, Cao J, Huang H, et al. Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period. J Neuroinflammation. 2017;14:6.
Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol. 2005;25:10286–300.
Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry. 2018;23:1134–44.
DeSteno DA, Schmauss C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience. 2008;152:417–28.
Poirier R, Cheval H, Mailhes C, Charnay P, Davis S, Laroche S. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Front Behav Neurosci. 2007;1:6.
Liu L, Zhu J, Glass PS, Brink PR, Rampil IJ, Rebecchi MJ. Age-associated changes in cardiac gene expression after preconditioning. Anesthesiology. 2009;111:1052–64.
Baets J, Deconinck T, De Vriendt E, Zimon M, Yperzeele L, Van Hoorenbeeck K. et al. Genetic spectrum of hereditary neuropathies with onset in the first year of life. Brain. 2011;134:2664–76.
Adler SM, Schmauss C. Cognitive deficits triggered by early life stress: The role of histone deacetylase 1. Neurobiol Dis. 2016;94:1–9.
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–9.
Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiol Rev. 2014;94:141–88.
Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–56.
Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–13.
Vihma H, Luhakooder M, Pruunsild P, Timmusk T. Regulation of different human NFAT isoforms by neuronal activity. J Neurochem. 2016;137:394–408.
Mabb AM, Ehlers MD. Arc ubiquitination in synaptic plasticity. Semin Cell Dev Biol. 2018;77:10–6.
Zhang F, Zhu Q, Xue Q, Luo Y, Yu B. Extra-cellular signal-regulated kinase (ERK) is inactivated associating hippocampal ARC protein up-regulation in sevoflurane induced bidirectional regulation of memory. Neurochem Res. 2013;38:1341–7.
Song SY, Meng XW, Xia Z, Liu H, Zhang J, Chen QC, et al. Cognitive impairment and transcriptomic profile in hippocampus of young mice after multiple neonatal exposures to sevoflurane. Aging (Albany NY). 2019;11:8386–417.
Mengozzi M, Cervellini I, Villa P, Erbayraktar Z, Gokmen N, Yilmaz O, et al. Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc Natl Acad Sci. 2012;109:9617–22.
Kim SH, Song JY, Joo EJ, Lee KY, Shin SY, Lee YH, et al. Genetic association of the EGR2 gene with bipolar disorder in Korea. Exp Mol Med. 2012;44:121–9.
Maeda N, Kawakami S, Ohmoto M, le Coutre J, Vinyes-Pares G, Arigoni F, et al. Differential expression analysis throughout the weaning period in the mouse cerebral cortex. Biochem Biophys Res Commun. 2013;431:437–43.
Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, et al. Tau contributes to sevoflurane-induced neurocognitive impairment in neonatal mice. Anesthesiology. 2020;133:595–610.
Cao X, Ma Q, Wang B, Qian Q, Liu N, Liu T, et al. Silencing long non-coding RNA MIAT ameliorates myocardial dysfunction induced by myocardial infarction via MIAT/miR-10a-5p/EGR2 axis. Aging (Albany NY). 2021;13:11188–206.
Ghi P, Di Brisco F, Dallorto D, Osella MC, Orsetti M. Age-related modifications of egr1 expression and ubiquitin-proteasome components in pet dog hippocampus. Mech Ageing Dev. 2009;130:320–7.
Koldamova R, Schug J, Lefterova M, Cronican AA, Fitz NF, Davenport FA, et al. Genome-wide approaches reveal EGR1-controlled regulatory networks associated with neurodegeneration. Neurobiol Dis. 2014;63:107–14.
Ravassard P, Hamieh AM, Joseph MA, Fraize N, Libourel PA, Lebarillier L, et al. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex. 2016;26:1488–500.
Nishimura Y, Takizawa R, Koike S, Kinoshita A, Satomura Y, Kawasaki S, et al. Association of decreased prefrontal hemodynamic response during a verbal fluency task with EGR3 gene polymorphism in patients with schizophrenia and in healthy individuals. NeuroImage. 2014;85:527–34.
Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, et al. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci. 2007;35:76–88.
Gallitano-Mendel A, Wozniak DF, Pehek EA, Milbrandt J. Mice lacking the immediate early gene Egr3 respond to the anti-aggressive effects of clozapine yet are relatively resistant to its sedating effects. Neuropsychopharmacology. 2008;33:1266–75.
Cheng MC, Chuang YA, Lu CL, Chen YJ, Luu SU, Li JM, et al. Genetic and functional analyses of early growth response (EGR) family genes in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:149–55.
Poirier R, Cheval H, Mailhes C, Garel S, Charnay P, Davis S, et al. Distinct functions of egr gene family members in cognitive processes. Front Neurosci. 2008;2:47–55.
Gonzalez-Gutierrez A, Lazo OM, Bronfman FC. The Rab5-Rab11 endosomal pathway is required for BDNF-induced CREB transcriptional regulation in hippocampal neurons. J Neurosci. 2020;40:8042–54.
Zhao H, Alam A, San CY, Eguchi S, Chen Q, Lian Q, et al. Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: Recent developments. Brain Res. 2017;1665:1–21.
Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103.
von Bohlen Und Halbach O, von Bohlen Und Halbach V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018;373:729–41.
Hatano R, Ohnuma K, Otsuka H, Komiya E, Taki I, Iwata S, et al. CD26-mediated induction of EGR2 and IL-10 as potential regulatory mechanism for CD26 costimulatory pathway. J Immunol. 2015;194:960–72.
Wang J, Zhang Y, Liu L, Cui Z, Shi R, Hou J, et al. NFAT2 overexpression suppresses the malignancy of hepatocellular carcinoma through inducing Egr2 expression. BMC Cancer. 2020;20:966.
Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–69.
Ni C, Li Z, Qian M, Zhou Y, Wang J, Guo X. Isoflurane induced cognitive impairment in aged rats through hippocampal calcineurin/NFAT signaling. Biochem Biophys Res Commun. 2015;460:889–95.
Li Y, Chen D, Wang H, Wang Z, Song F, Li H, et al. Intravenous versus volatile anesthetic effects on postoperative cognition in elderly patients undergoing laparoscopic abdominal surgery. Anesthesiology. 2021;134:381–94.
Fei X, Wang JX, Wu Y, Dong N, Sheng ZY. Sevoflurane-induced cognitive decline in aged mice: Involvement of toll-like receptors 4. Brain Res Bull. 2020;165:23–9.
Chen Y, Zhang P, Lin X, Zhang H, Miao J, Zhou Y, et al. Mitophagy impairment is involved in sevoflurane-induced cognitive dysfunction in aged rats. Aging (Albany NY). 2020;12:17235–56.
Liu J, Li L, Xie P, Zhao X, Shi D, Zhang Y, et al. Sevoflurane induced neurotoxicity in neonatal mice links to a GSK3beta/Drp1-dependent mitochondrial fission and apoptosis. Free Radic Biol Med. 2022;181:72–81.
Zhang Y, Lu P, Liang F, Liufu N, Dong Y, Zheng JC, et al. Cyclophilin D contributes to anesthesia neurotoxicity in the developing brain. Front Cell Dev Biol. 2019;7:396.
Zhao S, Fan Z, Hu J, Zhu Y, Lin C, Shen T, et al. The differential effects of isoflurane and sevoflurane on neonatal mice. Sci Rep. 2020;10:19345.
Graber TG, Fandrey KR, Thompson LV. Novel individualized power training protocol preserves physical function in adult and older mice. GeroScience. 2019;41:165–83.
Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30:329–42.
Acknowledgements
Thanks for the technical support by the Core Facilities, Zhejiang University School of Medicine. The library preparations were sequenced on an Illumina Novaseq6000 platform and paired-end reads were generated, and bioinformatics analysis (Shanghai Origingene Bio-pharm Technology Co.Ltd., China). This research was supported by the National Natural Science Foundation of China (No.82171176 and No.82001424), the Zhejiang Provincial Natural Science Foundation of China (No. LQ19H030009), and the Key Program of the Natural Science Foundation of Zhejiang, China (No. LZ19H090003).
Author information
Authors and Affiliations
Contributions
YRC and GC participated in the design of the experimental protocols. YRC, SXZ, MF, and YFZ carried out experimental operation. YRC, PZ, and XY were in charge of the data analysis. YRC and XNZ drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, Yr., Zhang, Sx., Fang, M. et al. Egr2 contributes to age-dependent vulnerability to sevoflurane-induced cognitive deficits in mice. Acta Pharmacol Sin 43, 2828–2840 (2022). https://doi.org/10.1038/s41401-022-00915-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00915-5
Keywords
This article is cited by
-
Lipocalin 2 in the Paraventricular Thalamic Nucleus Contributes to DSS-Induced Depressive-Like Behaviors
Neuroscience Bulletin (2023)