Abstract
Transcription factors (TFs) specifically bind to DNA, recruit cofactor proteins and modulate target gene expression, rendering them essential roles in the regulation of numerous biological processes. Meanwhile, mutated or dysregulated TFs are involved in a variety of human diseases. As multiple signaling pathways ultimately converge at TFs, targeting these TFs directly may prove to be more specific and cause fewer side effects, than targeting the upfront conventional targets in these pathways. All these features together endue TFs with great potential and high selectivity as therapeutic drug targets. However, TFs have been historically considered “undruggable”, mainly due to their lack of structural information, especially about the appropriate ligand-binding sites and protein-protein interactions, leading to relatively limited choices in the TF-targeting drug design. In this review, we summarize the recent progress of TF-targeting drugs and highlight certain strategies used for targeting TFs, with a number of representative drugs that have been approved or in the clinical trials as examples. Various approaches in targeting TFs directly or indirectly have been developed. Common direct strategies include aiming at defined binding pockets, proteolysis-targeting chimaera (PROTAC), and mutant protein reactivation. In contrast, the indirect ones comprise inhibition of protein-protein interactions between TF and other proteins, blockade of TF expression, targeting the post-translational modifications, and targeting the TF-DNA interactions. With more comprehensive structural information about TFs revealed by the powerful cryo-electron microscopy technology and predicted by machine-learning algorithms, plus more efficient compound screening platforms and a deeper understanding of TF-disease relationships, the development of TF-targeting drugs will certainly be accelerated in the near future.
Similar content being viewed by others
Introduction
Transcription factors (TFs) recognize specific DNA sequences on chromatin and recruit a complex transcriptional machinery to decode the genome, which harbors over 1600 TFs among the 24,000 protein-encoding genes in human cells [1, 2]. It is essential that TFs play their roles properly as part of the cross-talking and dynamic TF network [1]. Usually TFs are expressed in a cell type-specific manner to coordinate the gene expression programs underlying a vast array of cellular processes [3]. Many TFs function as “master regulators”, exerting control over processes of the development and differentiation in eukaryotic systems that require time- and tissue-specific transcriptional programs [4]. In the past decades, evidence has mounted that TFs are critical drivers of human diseases [5], such as cancer, cardiovascular diseases, diabetes, obesity, and neurodegenerative diseases [6]. In tumor cells, genes encoding TFs are often amplified, deleted, rearranged via chromosomal translocation, or subjected to point mutations that result in a gain- or loss-of-function [3]. A classic example is the tumor suppressor p53, which is known to be dysregulated in more than half of all human cancers [7].
The functional significance of TFs in many biological processes and their aberrant activities in human diseases, together highlight their potentials as therapeutic drug targets. However, TFs were historically viewed as “undruggable” [8]. In contrast to kinases or other target enzymes [9], which have measurable enzymatic activities and associated binding pockets that small-molecule drugs can be directed to [10], most TFs usually do not exhibit defined ligand-binding pockets, with a few exceptions as described below. In addition, TFs mainly act on protein-protein and protein-DNA interactions, which are usually transient and often accompanied by relatively large and flat interfaces. As a result, the design of small-molecule compounds aiming to interrupt or enhance these TF-related interactions selectively by direct binding, becomes a tough task. Moreover, the typically convex and highly positively charged DNA binding interfaces of TFs, also make the development of small-molecule inhibitors with drug-like properties targeting these areas very much challenging [9].
Due to the fact that signaling pathways ultimately converge at TFs, targeting these TFs directly may prove to be more specific and cause less side effects, than targeting the upfront conventional targets in these pathways. Joint efforts from both academia and industry have focused on the development of small-molecule drugs that would specifically target a particular TF. With the insights into protein structures and the understanding of biochemical and biomedical properties of TFs, many “undruggable” TFs are successfully targeted, and a considerable number of small molecules have been approved or have entered clinical trials (Table 1). In this review, we classify TF-targeting strategies roughly into two groups, “directly” or “indirectly”, and summarize the current progress of TF-targeting drugs using these strategies with several typical examples.
Directly targeting TF
For most of TFs, it is hard to be targeted directly by small molecules, due to their lack of a defined binding pocket. The nuclear receptors (NRs) and the basic helix-loop-helix-PER-ARNT-SIM (bHLH-PAS) proteins are two exceptional TF families that usually possess an architecture harboring poteintial ligand-binding sites, which not only interact with ligands but also exert the consequent modulations of TF activities. In addition, covalent binding by small molecules to reactivate certain TF mutants is also an effective strategy to target TFs directly.
Targeting a defined binding pocket
Among the >1600 human TFs, the family of NRs containing only 48 members, provide the most druggable targets that have achieved great success in clinical. About 16% of all the small-molecule drugs approved by the US Food and Drug Administration (FDA) are believed to target NRs, ranking the third after the other two well-known groups of drug targets, G-protein coupled receptors (GPCRs, 33%) and ion channels (18%) [11]. NRs are a family of ligand-regulated TFs that possess a DNA-binding domain (DBD) and a ligand-binding domain (LBD). The binding of small-molecule ligands induces conformational changes of NRs, which can modulate their interactions with various transcriptional coregulators (such as the coactivators or corepressors), thus controlling the expression of downstream target genes [12]. The activities of NRs can be modulated by small molecules that mimic their natural ligands, making them ideal therapeutic targets. In addition, the structural information for most of NR LBDs and a few of full-length NR complexes, has been revealed in the past three decades [13], and greatly facilitated the NR-targeting drug discovery. Accordingly, a vast array of NR drugs and chemical probes have been developed.
To delineate how NRs can be directly targeted, we take a representative NR, androgen receptor (AR) as an example (Fig. 1). Binding of androgens to AR LBD leads to the nucleus translocation and initiates target gene expression [14]. AR signaling has been proven as a critical driver in prostate cancers, making AR an essential therapeutic target [15, 16]. To date, there have been two generations of nonsteroidal AR antagonists approved in clinical to treat prostate cancer [16]. Enzalutamide is the first second-generation AR antagonist with more potent efficacy compared with the first-generation drugs, and has been approved by US FDA to treat three forms of advanced prostate cancers [16]. Besides, a number of AR antagonists are undergoing clinical trials, such as proxalutamide and EPI-7386. To meet the need of drugs that can exert anabolic effects of androgens on muscle and bone through AR signaling pathway and avoid undesirable side effects, the selective AR modulators (SARMs) were synthesized and developed over the past two decades [17]. Among them, GTx-024 is a first-in-class SARM developed for cancer and stress urinary incontinence in women [18].
Schematic representation showing the regulation of AR downstream genes by small-molecule modulators. a Androgens or SARMs bind to AR LBD and induce conformational changes that facilitate the recruitment of coactivators and activation of gene expression. b The binding of antagonists to LBD blocks the AR signaling pathway. c, d The binding of PROTAC molecules to AR LBD (c) or DBD (d) promotes proteasome-mediated degradation of AR and decreases the activity of AR signaling pathway.
Despite the NR family being most druggable among TFs, targeting all family members of NRs is still a very challenging task. For instance, some NRs especially those called orphan receptors, do not have assigned endogenous ligands or obvious ligand-binding pockets [19]. Besides, the expression of NR splice variants that lack the LBDs in some diseases can render traditional drugs ineffective, such as for the AR-V7, a splice variant of AR [20, 21]. Traditional NR-targeting drugs all bind to the LBD pockets. More recently, NR coactivator binding inhibitors have also been developed to modulate NR activities [22].
In addition to NRs, the bHLH-PAS family has been identified as the second TF family that possess potential ligand-binding pockets within their PAS domains and thus may serve as a new group of drug targets [23, 24]. One representative member of this family is the hypoxia-inducible factor 2α (HIF-2α), which contains a defined binding pocket of about 300 Å3 in the PAS-B domain [25]. HIF-2α dimerizes with its obligate partner, the HIF-β subunit also called aryl hydrocarbon receptor nuclear translocator (ARNT), to form a transcriptionally active heterodimer [26]. Under normoxia, HIF-prolyl hydroxylase domain (PHD) proteins conduct the hydroxylation of Pro405 and Pro531 in the oxygen-dependent degradation domain (ODDD) of HIF-2α, leading to the subsequent ubiquitination and proteasomal degradation (Fig. 2) [27]. Meanwhile, within the C-terminal transactivation domain (CTAD) of HIF-2α, Asn847 can also be hydroxylated by the factor inhibiting HIF (FIH) enzyme, further decreasing HIF-2α activity by blocking its interactions to coactivators [28]. Under hypoxia, the oxygen-dependent PHD and FIH enzymes are deactivated, allowing HIF-2α’s accumulation and translocation to promote the transcription of gene programs closely associated with angiogenesis, erythropoiesis, cell proliferation, and migration [26].
Under normoxia, HIF-2α protein is hydroxylated and degraded to maintain a low level. Under hypoxia, HIF-2α accumulates and dimerizes with ARNT to active target gene expression. PHD inhibitors block HIF-2α degradation and activate the HIF-2α signaling pathway. The binding of an antagonist disrupts HIF-2α/ARNT interaction and blocks the HIF-2α signaling pathway. The binding of an agonist enhances HIF-2α/ARNT interaction and activates target gene expression.
The tumor suppressor von Hippel-Lindau protein (pVHL) is a component of the E3 ubiquitin-ligase complex that mediates HIF-2α degradation [29, 30]. Approximately 90% of the patients with clear cell renal cell carcinoma (ccRCC) exhibit pVHL inactivation, resulting in an excessive level of HIF-2α proteins [31]. Moreover, HIF-2α has been identified as a key oncogenic driver in ccRCC, rendering it a promising drug target for this type of kidney cancer [27, 32]. The first crystal structure of the HIF-2α PAS-B domain revealed a water-bound internal cavity that potentially accepts artificial ligands [33], and thus initiated a successful campaign of structure-based drug discovery that has transformed from bench to bedside. PT2385, a small-molecule compound that can bind into the PAS-B pocket of HIF-2α and disrupt HIF-2α/ARNT interactions through allosteric effects, is the first HIF-2α antagonist entering clinical trials [34]. Belzutifan (PT2977/MK-6482) is an upgraded version of HIF-2α antagonist with improved pharmacokinetic profiles [35] and it obtained the approval from FDA in August 2021 for the treatment of cancers associated with the VHL disease, including ccRCC. In addition, the function of HIF pathway may be suppressed in patients with chronic kidney disease (CKD), in contrast to the situation in patients with ccRCC [36]. Enhancing the activity of HIF-2α to increase erythropoietin (EPO) expression and iron metabolism could ameliorate renal anemia in CKD [37]. The recently discovered HIF-2α agonists bind into the same PAS-B pocket as antagonists, yet allosterically causing stabilizing effects on the HIF-2α-ARNT heterodimer [38]. Overall, bidirectional regulation of HIF-2α activity by small-molecule ligands has a great potential in the treatment of various diseases driven by or related to HIF-2α.
PROTAC
In recent years, proteolysis-targeting chimera (PROTAC) technology has been developed and turned out to be a potential therapeutic method to treat various diseases caused by protein overexpression [39, 40]. A PROTAC molecule consists of a ligand (mostly small-molecule inhibitor) for the protein of interest (POI), a ligand of an E3 ubiquitin ligase (E3), and a linker region covalently connecting the two ligands. Upon binding to POI, the PROTAC molecule can recruit E3 for proximity-induced ubiquitination of POI, which is then subjected to degradation by endogenous 26S proteasome. PROTAC only requires a ligand that binds to the POI, which may not necessarily affect POI’s function. In addition, the catalytic nature of PROTAC suggests that it requires lower drug exposure to achieve certain efficacy. Based on the mechanism of action, PROTAC can potentially target any type of protein, including the previously “undruggable” class of therapeutic protein targets [41].
The ability of PROTAC molecules to degrade TFs, scaffolding proteins, and other proteins without an enzymatic function would greatly expand the druggable proteome [42]. The relatively simple chemical structure of PROTAC allows the rapid conversion of protein ligands into effective degraders, potentially making the target proteome susceptible to therapeutic intervention. Furthermore, elucidation of a target-PROTAC-ligase ternary structure could be essential for the design of PROTAC molecules based on ligands with weak binding affinities to target proteins [40]. PROTAC technology has been successfully used to design compounds that mediate the degradation of estrogen receptors (ER), as well as AR [43]. ARV-471, an ERα-targeting PROTAC developed by Arvinas Inc, is now in phase II clinical study to treat patients with ER positive/human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer. ARV-110, also developed by Arvinas Inc, is an AR-targeting PROTAC undergoing clinical trials to treat metastatic castration-resistant prostate cancer (Fig. 1). Recently, Geun Taek Lee et al. reported MTX-23, which can target both AR-V7 splice variant and full-length AR protein by binding to AR’s DBD rather than its LBD [44]. This PROTAC molecule has shown a good anti-tumor activity both in vitro and in vivo (Fig. 1).
Mutant protein reactivation
Mutations are often correlated with the dysfunction of TFs in various biological processes. Usually, the conformational changes of mutated proteins will result in impairment or loss of their functions. The TF p53 is a widely investigated and desirable target for cancer treatment, as it is found to be inactivated in most of human malignancies [45]. Mutation and its abnormal interaction with murine double minute 2 (MDM2)/murine double minute 4 (MDM4) are the two main mechanisms for p53 inactivation [45, 46]. The TP53 gene encoding the p53 protein is the most frequently mutated gene in human cancers [47]. Missense mutations account for 75% of all TP53 mutations in cancer and result in the loss of wild-type (WT) function [45, 47]. Among the missense mutations, R175, G245, R248, R273, and R282 are the five most frequently affected residues located in the DBD [48]. Targeting p53 mutants to restore their normal function is a promising strategy for tumors with mutated p53 [48]. p53 mutants have been viewed as “undruggable” for various reasons, such as lack of binding pockets for small molecules and their subcellular location in the nucleus [45]. Despite all of this, a number of small molecules that can reactivate p53 mutants have emerged over the past two decades, of which arsenic trioxide, APR-246, and COTI-2 have progressed to clinical evaluations.
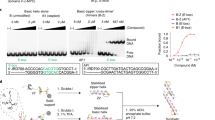
Arsenic trioxide has been used to treat acute promyelocytic leukemia clinically by targeting promyelocytic leukemia/retinoic acid receptor alpha (PML-RARα) [49]. A recent study reported that arsenic trioxide could stabilize p53 folding and reactivate p53 mutants for tumor suppression by its binding to p53 cysteine residues covalently [50]. The therapeutic effects of arsenic trioxide in refractory cancer patients with p53 mutations are evaluated in different clinical trials.
APR-246 is a prodrug that is subsequently converted to methylene quinuclidinone (MQ) through hydrolysis [48]. MQ binds to the cysteines covalently in the p53 DBD and induces the refolding of mutated p53 to WT conformation [51]. For the mutant R175H, Cys124 and Cys277 are identified as essential target residues for APR-246 mediated reactivation [51]. The clinical safety and efficacy of APR-246 have been investigated preliminarily, and it is currently undergoing a clinical trial in phase III in patients with TP53 mutation-related myelodysplastic syndromes.
Another p53-reactivating compound in clinical trials is COTI-2, a thiosemicarbazone derivative. Surface plasmon resonance (SPR) assays showed that COTI-2 could bind to p53 mutants directly, and flow cytometry assays indicated that it could efficiently induce apoptosis in p53-mutated cell lines such as BT549, Hs578T, and CAMA1 [52]. COTI-2 is currently undergoing a phase I clinical trial to assess the safety and tolerability in patients with advanced and recurrent malignancies.
Indirectly targeting TF
Targeting PPI of TFs
Protein-protein interactions (PPIs) play essential roles in numerous physiological processes and are often dysregulated in diseases. Therefore, PPIs have also been considered as potential therapeutic targets [53]. Mutational analysis of protein surfaces reveal that usually a limited number of key residues contribute to most of the binding energy, suggesting that inhibition of PPIs by small molecules is feasible [54]. Generally, TFs interact with other proteins (such as different TFs, coactivators or corepressors) and bind to the specific DNA sequences to regulate the expression of target genes. A number of TF modulators have been developed by targeting PPIs recently [9, 55].
For tumors retaining a WT p53, studies are mostly focused on blocking interactions between p53 and MDM2, MDM4 or both proteins to enhance the p53 protein level. The development of MDM2 inhibitors that can interrupt the MDM2-p53 interaction has achieved great progress, along with the disclosed details from the co-crystal structures of MDM2-p53 and MDM2-Nutlin-2 [56, 57]. Several small molecules that can mimic the interaction between p53 and MDM2 are in different stages of clinical trials as MDM2 inhibitors, such as BI-907828 [58], RG7112 [59], RG7388 [60], NVP-CGM097 [61], AMG232 [62], SAR405838 [63], DS-3032b [64], APG-115 [65] and HDM201 [66]. The crystal structure of MDM4-p53 complex shows that the p53-binding pocket of MDM4 is smaller and differently shaped compared with the p53-binding pocket of MDM2 [67]. To date, only a few MDM4 inhibitors have been developed. The stapled peptide ALRN-6924 developed by Aileron Therapeutics is currently in several clinical trials, acting as an MDM2/MDM4 dual inhibitor [68]. Blocking MDM2-p53 interaction by MDM2 PROTAC degraders is another attractive strategy. The compound MD-224 was designed by conjugating MDM2 inhibitor MI-1061 to a cereblon E3 ligase ligand, and showed more effective effects on tumor regression compared with MI-1061 [69].
Another similar example is to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) by targeting its PPI with Kelch-like ECH-associated protein 1 (Keap1), a component of the Culins 3-based E3 ligase that can mediate the degradation of Nrf2 [70]. Under normal conditions, Nrf2 is in the cytoplasm and interacts with Keap1 to maintain a low protein level [71]. Under stress conditions, Keap1 is modified covalently by oxidative and electrophilic insults, resulting in nuclear translocation of Nrf2 and subsequent activation of the Nrf2 signaling pathway [72]. Nrf2 regulates the expression of more than two hundred genes that can protect the human body against oxidation and inflammation-related diseases, such as neurodegenerative diseases [73,74,75]. With the elucidation of the co-crystal structures of Keap1 and Nrf2 peptides, a large number of Keap1-Nrf2 inhibitors targeting their PPI have been reported, including small molecules and peptides [76, 77]. Among these PPI inhibitors, bardoxolone methyl is undergoing several clinical trials, and its New Drug Application (NDA) was accepted by FDA for the treatment of CKD caused by Alport syndrome in April 2021.
Blockade of TF expression
Among the multitudinous TFs, MYC oncoproteins (C-MYC, L-MYC, and N-MYC) are regarded as the most desirable and intractable targets for treating cancers [15, 78]. MYC proteins regulate over 2000 genes related to cell growth, differentiation, proliferation, apoptosis, angiogenesis, DNA repair, metabolism, and stem cell formation [79,80,81]. Dysregulation of MYC expression occurs in up to 70% of human cancers by many different mechanisms, such as altered protein stability, gene amplification, chromosomal translocation, mutation, and loss of p53 [82,83,84,85,86,87,88]. Given its crucial role in cancer formation, progression, and maintenance, MYC is recognized as an ideal target for treating malignancy [89]. However, MYC has been traditionally considered as undruggable due to the lack of defined binding pocket, intrinsically disordered structure, nuclear localization, and its essential function in multiple physiological regulations [78, 79]. Despite these challenges, several strategies targeting MYC to treat cancers have been developed in recent years.
One of the well-studied strategies is to inhibit MYC expression by epigenetic regulation. Bromodomain-containing protein 4 (BRD4), as a chromatin reader, can interact with a large number of TFs directly [90]. Besides, BRD4 can bind to acetylated nucleosomes located in the vicinity of TF-occupied enhancers and promoters to boost TF-mediated activation without direct TF interactions [90]. BRD4 inhibitors have been shown to downregulate the expression of MYC and exhibited anti-tumor activity in various animal models [91,92,93]. To date, several small molecules, including GSK525762, CPI-0610, ABBV-075, and AZD5153 that target BRD4 have advanced into clinical trials to treat hematological and solid tumors [94].
Besides the application of small-molecule compounds, various nucleic acid-based strategies have also been developed to target the expression of TFs. For example, siRNA molecules have been used to inhibit MYC expression as well as some other TFs, such as the signal transducers and activators of transcription 3 (STAT3) and HIF-1α [95, 96]. In addition, the cutting-edge CRISPR/Cas technology is also employed to target TFs by gene editing (deletion) for the treatment of certain diseases [97]. Interestingly, recent studies have shown that multiple highly structured mRNAs possess pockets with properties suitable for small molecules recognition, providing a new strategy for targeting the expression of TFs [98].
Targeting the PTMs of TF
The post-translational modifications (PTMs) such as ubiquitination, hydroxylation, methylation, acetylation, and phosphorylation, play pivotal roles in orchestrating the TF activities from various aspects, including their subcellular localization, protein stability, protein-protein interactions and sequence-specific DNA binding [99]. Therefore, these multiple PTMs of TFs provide a large number of potential therapeutic targets for the treatment of various diseases [100].
The Janus kinase (JAK)/STAT signaling pathway mediates various cellular processes, including cell proliferation, inflammatory response, stem cell maintenance, and differentiation [101]. STATs phosphorylated by JAKs subsequently translocate into the nucleus and mediate downstream gene transcription. Aberrant activation of the JAK/STAT pathway has been observed in different immune-mediated diseases and cancers [102, 103]. Since JAKs act upstream of STATs along the signaling axis, targeting JAKs to block the JAK/STAT signaling pathway is feasible. The JAK inhibitors, baricitinib, filgotinib, tofacitinib and upadacitinib have been approved to treat rheumatoid arthritis, while fedratinib and ruxolitinib have been approved for the treatment of myelofibrosis.
As mentioned above, another representative PTM-targeting strategy is the inhibition of HIF-α hydroxylation by the PHD enzymes, which is an indirect way to activate the HIF-2α signaling pathway (Fig. 2). PHD inhibitors have been developed mainly by the structure-based drug design (SBDD) method and inhibit PHD catalytic activities by binding to the ferrous-iron-containing active site [104]. Roxadustat is the first approved PHD inhibitor, which was launched in China in 2018 to treat renal anemia in CKD. In August 2021, roxadustat was also approved by European Commission. Other PHD inhibitors, including daprodustat, vadadustat, enarodustat and molidustat have also been approved in Japan with the same indication as roxadustat.
Targeting interactions between TF and DNA
DNA alkylating drugs have been used in clinical to treat cancers for almost 70 years, as the first class of drugs targeting DNA [105]. In the past two decades, many new advances have been made in the development of small molecules that can bind to DNA specifically to regulate TF activities [106]. For example, Hiroshi Sugiyama’s group recently developed a novel class of artificial TF-mimicking chemicals based on pyrrole-imidazole polyamides, which could target specific DNA sequences and regulate gene expressions [107]. However, to date no DNA-binding small molecule as the regulator of TF has been approved due to its poor specificity.
Conclusion and perspective
The development of TF-targeting drugs has been a successful achievement in the past decades, with multiple small-molecule drugs being approved or entering clinical trials. However, targeting TFs selectively and efficiently is still full of challenges. One of the reasons is the insufficiency of structural information of TFs, especially for those containing intrinsically disordered regions [108]. Since TFs are generally targeted by small-molecule chemicals (rather than large-molecule biologicals) due to their intracellular localization, it is crucial to obtain three-dimensional structures of TFs and identify proper ligand-binding sites for the design and discovery of drugs directly targeting those TFs. X-ray crystallography has produced numerous TF protein structures since 1950s. It has been a dominant tool to get protein structures until the advent of cryo-electron microscopy (cryo-EM). Although the most comprehensive structures of full-length ER or AR in complex with their large coactivators solved by cryo-EM are currently suffering from the low resolutions (>10 Å) [109,110,111], it is still very hopeful to reveal the detailed structural basis of how TFs interact with coactivators, corepressor or other partners in the near future, possibly by the improved sample preparation and upgraded microscopy. In addition to the experimental approaches to solve protein structures, machine-learning algorithms have made a significant breakthrough in determining a protein’s structure based on its amino-acid sequence. AlphaFold2, developed by Google AI offshoot DeepMind, has recently demonstrated very high accuracy in protein structure modeling [112, 113]. Meanwhile, David Baker’s group developed a deep-learning approach-based algorithm called RoseTTAFold to generate structure models [114]. As for the characterization of intrinsically disordered proteins, it is usually more suitable to combine NMR spectroscopy and molecular dynamics approaches [115,116,117]. All these above techniques are still more or less evolving to better illustrate the structural pictures of TFs, providing more information and even guidance to the future design of drugs targeting TFs directly or indirectly.
Another challenge comes from the lack of efficient screening platforms and functional assays for TFs. Unlike enzymes whose activities often can be tested biochemically, the transcriptional activities of TFs usually can only be measured in a cellular system by approaches such as qPCR of target genes and luciferase reporter assays. Therefore, traditionally the majority of high-throughput screenings for TF-targeting small-molecule drugs are transcription activity-based cellular platforms, which tend to show a relatively high off-target rate and often need further confirmation on the mechanism of action for hit compounds. As a result, a variety of biochemical screening strategies based on direct interactions have been widely adopted to discover new TF modulators with high throughput in recent years, such as the thermal shift assay [118], affinity selection-mass spectrometry (AS-MS) [119], SPR [120], and small molecule microarrays [121]. In addition, the incorporation of DNA-encoded libraries (DELs) in screenings has been an emerging technology [122]. It is noteworthy that the positive compounds obtained from the above direct interaction-based screenings need to be tested again using proper functional assays for their abilities to modulate TF activities. Nevertheless, compounds binding to definite TF targets can also be used in the development of PROTAC molecules.
Finally, a comprehensive understanding of the physiological functions of TFs, especially their causal relationships with a specific disease, is crucial for the drug development. Given the complexity of multiple signaling pathways converging at TFs, the optimal strategy to target a specific TF directly or indirectly has to be chosen in the context of potential crosstalk between pathways. Small-molecule modulators of TFs or related proteins in their pathways are very useful and convenient tools to help explore TF functions. Since many NR-targeting drugs are derivatives of endogenous NR ligands, identifying new intrinsic cellular ligands of TFs would be a key breakthrough for both functional study and drug design. Along with the knowledge accumulation of TF biological functions and technology advancement of hit compound discovery, more previously “undruggable” TFs will become amenable targets for new “first-in-class” drugs.
References
Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, et al. The human transcription factors. Cell. 2018;172:650–65.
International Human Genome, Sequencing C. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–45.
Bhagwat AS, Vakoc CR. Targeting transcription factors in cancer. Trends Cancer. 2015;1:53–65.
Singh H, Khan AA, Dinner AR. Gene regulatory networks in the immune system. Trends Immunol. 2014;35:211–8.
Papavassiliou KA, Papavassiliou AG. Transcription factor drug targets. J Cell Biochem. 2016;117:2693–6.
Wiedemann B, Weisner J, Rauh D. Chemical modulation of transcription factors. Medchemcomm. 2018;9:1249–72.
Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13:217–36.
Groner B, Weber A, Mack L. Increasing the range of drug targets: interacting peptides provide leads for the development of oncoprotein inhibitors. Bioengineered. 2012;3:320–5.
Bushweller JH. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer. 2019;19:611–24.
Gayvert K, Elemento O. Drug-induced expression-based computational repurposing of small molecules affecting transcription factor activity. Methods Mol Biol. 2019;1903:179–84.
Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19–34.
Koehler AN. A complex task? Direct modulation of transcription factors with small molecules. Curr Opin Chem Biol. 2010;14:331–40.
Khorasanizadeh S, Rastinejad F. Visualizing the architectures and interactions of nuclear receptors. Endocrinology. 2016;157:4212–21.
Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7:a030452.
Dang CV, Reddy EP, Shokat KM, Soucek L. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer. 2017;17:502–8.
Rajaram P, Rivera A, Muthima K, Olveda N, Muchalski H, Chen QH. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448.
Simitsidellis I, Esnal-Zuffiaure A, Kelepouri O, O’Flaherty E, Gibson DA, Saunders PTK. Selective androgen receptor modulators (SARMs) have specific impacts on the mouse uterus. J Endocrinol. 2019;242:227–39.
Coss CC, Jones A, Dalton JT. Pharmacokinetic drug interactions of the selective androgen receptor modulator GTx-024(Enobosarm) with itraconazole, rifampin, probenecid, celecoxib and rosuvastatin. Invest New Drugs. 2016;34:458–67.
de Vera IMS. Advances in orphan nuclear receptor pharmacology: a new era in drug discovery. ACS Pharmacol Transl Sci. 2018;1:134–7.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11.
Vishnoi K, Viswakarma N, Rana A, Rana B. Transcription factors in cancer development and therapy. Cancers (Basel). 2020;12:2296.
Wu D, Su X, Potluri N, Kim Y, Rastinejad F. NPAS1-ARNT and NPAS3-ARNT crystal structures implicate the bHLH-PAS family as multi-ligand binding transcription factors. Elife. 2016;5:e18790.
Wu D, Rastinejad F. Structural characterization of mammalian bHLH-PAS transcription factors. Curr Opin Struct Biol. 2017;43:1–9.
Wu D, Potluri N, Lu J, Kim Y, Rastinejad F. Structural integration in hypoxia-inducible factors. Nature. 2015;524:303–8.
McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–45.
Yu Y, Yu Q, Zhang X. Allosteric inhibition of HIF-2alpha as a novel therapy for clear cell renal cell carcinoma. Drug Discov Today. 2019;24:2332–40.
Yan Q, Bartz S, Mao M, Li L, Kaelin WG Jr. The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–102.
Iliopoulos O, Levy AP, Jiang C, Kaelin WG Jr., Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–9.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5.
Melendez-Rodriguez F, Roche O, Sanchez-Prieto R, Aragones J. Hypoxia-inducible factor 2-dependent pathways driving von Hippel-Lindau-deficient renal cancer. Front Oncol. 2018;8:214.
Biswas S, Troy H, Leek R, Chung YL, Li JL, Raval RR, et al. Effects of HIF-1alpha and HIF2alpha on growth and metabolism of clear-cell renal cell carcinoma 786-0 Xenografts. J Oncol. 2010;2010:757908.
Scheuermann TH, Tomchick DR, Machius M, Guo Y, Bruick RK, Gardner KH. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc Natl Acad Sci USA. 2009;106:450–5.
Wehn PM, Rizzi JP, Dixon DD, Grina JA, Schlachter ST, Wang B, et al. Design and activity of specific hypoxia-inducible factor-2alpha (HIF-2alpha) inhibitors for the treatment of clear cell renal cell carcinoma: discovery of clinical candidate (S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1 H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J Med Chem. 2018;61:9691–721.
Xu R, Wang K, Rizzi JP, Huang H, Grina JA, Schlachter ST, et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzo nitrile (PT2977), a hypoxia-inducible factor 2alpha (HIF-2alpha) inhibitor for the treatment of clear cell renal cell carcinoma. J Med Chem. 2019;62:6876–93.
Tanaka T. Expanding roles of the hypoxia-response network in chronic kidney disease. Clin Exp Nephrol. 2016;20:835–44.
Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117:1926–32.
Wu D, Su X, Lu J, Li S, Hood BL, Vasile S, et al. Bidirectional modulation of HIF-2 activity through chemical ligands. Nat Chem Biol. 2019;15:367–76.
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–9.
Hu B, Zhou Y, Sun D, Yang Y, Liu Y, Li X, et al. PROTACs: new method to degrade transcription regulating proteins. Eur J Med Chem. 2020;207:112698.
Konstantinidou M, Li J, Zhang B, Wang Z, Shaabani S, Ter Brake F, et al. PROTACs- a game-changing technology. Expert Opin Drug Discov. 2019;14:1255–68.
Moon S, Lee BH. Chemically induced cellular proteolysis: an emerging therapeutic strategy for undruggable targets. Mol Cells. 2018;41:933–42.
Wang Y, Jiang X, Feng F, Liu W, Sun H. Degradation of proteins by PROTACs and other strategies. Acta Pharm Sin B. 2020;10:207–38.
Lee GT, Nagaya N, Desantis J, Madura K, Sabaawy HE, Kim WJ, et al. Effects of MTX-23, a novel PROTAC of androgen receptor splice variant-7 and androgen receptor, on CRPC resistant to second-line antiandrogen therapy. Mol Cancer Ther. 2021;20:490–9.
Duffy MJ, Synnott NC, O'Grady S, Crown J. Targeting p53 for the treatment of cancer. Semin Cancer Biol. 2020 Jul 31. https://doi.org/10.1016/j.semcancer.2020.07.005.
Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017;83:258–65.
Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–88.
Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102.
Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328:240–3.
Chen S, Wu JL, Liang Y, Tang YG, Song HX, Wu LL, et al. Arsenic trioxide rescues structural p53 mutations through a cryptic allosteric site. Cancer Cell. 2021;39:225–39 e228.
Zhang Q, Bykov VJN, Wiman KG, Zawacka-Pankau J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018;9:439.
Synnott NC, O’Connell D, Crown J, Duffy MJ. COTI-2 reactivates mutant p53 and inhibits growth of triple-negative breast cancer cells. Breast Cancer Res Treat. 2020;179:47–56.
Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem Biol. 2014;21:1102–14.
Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–17.
Maculins T, Garcia-Pardo J, Skenderovic A, Gebel J, Putyrski M, Vorobyov A, et al. Discovery of protein–protein interaction inhibitors by integrating protein engineering and chemical screening platforms. Cell Chem Biol. 2020;27:1441–51 e1447.
Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–53.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8.
Konopleva M, Martinelli G, Daver N, Papayannidis C, Wei A, Higgins B, et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34:2858–74.
Ray-Coquard I, Blay JY, Italiano A, Le Cesne A, Penel N, Zhi JG, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13:1133–40.
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56:5979–83.
Bauer S, Demetri GD, Halilovic E, Dummer R, Meille C, Tan DSW, et al. Pharmacokinetic-pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies. Br J Cancer. 2021;125:687–98.
Sun D, Li Z, Rew Y, Gribble M, Bartberger MD, Beck HP, et al. Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J Med Chem. 2014;57:1454–72.
de Jonge M, de Weger VA, Dickson MA, Langenberg M, Le Cesne A, Wagner AJ, et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur J Cancer. 2017;76:144–51.
Arnhold V, Schmelz K, Proba J, Winkler A, Wünschel J, Toedling J, et al. Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma. Oncotarget. 2017;9:2304–19.
Aguilar A, Lu J, Liu L, Du D, Bernard D, McEachern D, et al. Discovery of 4-((3'R,4'S,5'R)-6″-Chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-2″-oxodispiro[cyclohexane-1,2'-pyrrolidine-3',3″-indoline]-5'-carboxamido)bicyclo[2.2.2]octane-1-carboxylic acid (AA-115/APG-115): a potent and orally active murine double minute 2 (MDM2) inhibitor in clinical development. J Med Chem. 2017;60:2819–39.
Stein EM, DeAngelo DJ, Chromik J, Chatterjee M, Bauer S, Lin CC, et al. Results from a first-in-human phase I study of siremadlin (HDM201) in patients with advanced wild-type TP53 solid tumors and acute leukemia. Clin Cancer Res. 2021. https://doi.org/10.1158/1078-0432.CCR-21-1295.
Popowicz GM, Czarna A, Holak TA. Structure of the human Mdmx protein bound to the p53 tumor suppressor transactivation domain. Cell Cycle. 2008;7:2441–3.
Saleh MN, Patel MR, Bauer TM, Goel S, Falchook GS, Shapiro GI, et al. Phase 1 Trial of ALRN-6924, a dual inhibitor of MDMX and MDM2, in patients with solid tumors and lymphomas bearing wild-type TP53. Clin Cancer Res. 2021. https://doi.org/10.1158/1078-0432.CCR-21-0715.
Li Y, Yang J, Aguilar A, McEachern D, Przybranowski S, Liu L, et al. Discovery of MD-224 as a first-in-class, highly potent, and efficacious proteolysis targeting chimera murine double minute 2 degrader capable of achieving complete and durable tumor regression. J Med Chem. 2019;62:448–66.
Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44.
Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–900.
Sihvola V, Levonen AL. Keap1 as the redox sensor of the antioxidant response. Arch Biochem Biophys. 2017;617:94–100.
Raghunath A, Sundarraj K, Nagarajan R, Arfuso F, Bian J, Kumar AP, et al. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol. 2018;17:297–314.
Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–83.
Gao F, Li JM, Xi C, Li HH, Liu YL, Wang YP, et al. Magnesium lithospermate B protects the endothelium from inflammation-induced dysfunction through activation of Nrf2 pathway. Acta Pharmacol Sin. 2019;40:867–78.
Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25:3605–17.
Mou Y, Wen S, Li YX, Gao XX, Zhang X, Jiang ZY. Recent progress in Keap1-Nrf2 protein–protein interaction inhibitors. Eur J Med Chem. 2020;202:112532.
Whitfield JR, Beaulieu ME, Soucek L. Strategies to inhibit Myc and their clinical applicability. Front Cell Dev Biol. 2017;5:10.
Duffy MJ, O’Grady S, Tang M, Crown J. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94:102154.
Dang CV. A time for MYC: metabolism and therapy. Cold Spring Harb Symp Quant Biol. 2016;81:79–83.
Carroll PA, Freie BW, Mathsyaraja H, Eisenman RN. The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med. 2018;12:412–25.
Schick M, Habringer S, Nilsson JA, Keller U. Pathogenesis and therapeutic targeting of aberrant MYC expression in haematological cancers. Br J Haematol. 2017;179:724–38.
Xu-Monette ZY, Deng Q, Manyam GC, Tzankov A, Li L, Xia Y, et al. Clinical and biologic significance of MYC genetic mutations in de novo diffuse large B-cell Lymphoma. Clin Cancer Res. 2016;22:3593–605.
Schaub FX, Dhankani V, Berger AC, Trivedi M, Richardson AB, Shaw R, et al. Pan-cancer alterations of the MYC oncogene and its proximal network across the cancer genome atlas. Cell Syst. 2018;6:282–300 e282.
Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90.
Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35.
Kalkat M, De Melo J, Hickman KA, Lourenco C, Redel C, Resetca D, et al. MYC deregulation in primary human cancers. Genes (Basel). 2017;8:151.
Santoro A, Vlachou T, Luzi L, Melloni G, Mazzarella L, D’Elia E, et al. p53 loss in breast cancer leads to Myc activation, increased cell plasticity, and expression of a mitotic signature with prognostic value. Cell Rep. 2019;26:624–38 e628.
Hartl M. The quest for targets executing MYC-dependent cell transformation. Front Oncol. 2016;6:132.
Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54:728–36.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–17.
Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8.
Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–33.
Lu T, Lu W, Luo C. A patent review of BRD4 inhibitors (2013-2019). Expert Opin Ther Pat. 2020;30:57–81.
Kabilova TO, Chernolovskaya EL, Vladimirova AV, Vlassov VV. Silencing of c-myc expression in tumor cells by siRNA. Nucleosides Nucleotides Nucleic Acids. 2004;23:867–72.
Budi HS, Izadi S, Timoshin A, Asl SH, Beyzai B, Ghaderpour A, et al. Blockade of HIF-1alpha and STAT3 by hyaluronate-conjugated TAT-chitosan-SPION nanoparticles loaded with siRNA molecules prevents tumor growth. Nanomedicine. 2021;34:102373.
Riedel M, Cai H, Stoltze IC, Vendelbo MH, Wagner EF, Bakiri L, et al. Targeting AP-1 transcription factors by CRISPR in the prostate. Oncotarget. 2021;12:1956–61.
Hewitt WM, Calabrese DR, Schneekloth JS Jr. Evidence for ligandable sites in structured RNA throughout the Protein Data Bank. Bioorg Med Chem. 2019;27:2253–60.
Filtz TM, Vogel WK, Leid M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol Sci. 2014;35:76–85.
Qian M, Yan F, Yuan T, Yang B, He Q, Zhu H. Targeting post-translational modification of transcription factors as cancer therapy. Drug Discov Today. 2020;25:1502–12.
Svinka J, Mikulits W, Eferl R. STAT3 in hepatocellular carcinoma: new perspectives. Hepat Oncol. 2014;1:107–20.
Jamilloux Y, El Jammal T, Vuitton L, Gerfaud-Valentin M, Kerever S, Seve P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18:102390.
Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas). 2019;55:526.
Barrett TD, Palomino HL, Brondstetter TI, Kanelakis KC, Wu X, Haug PV, et al. Pharmacological characterization of 1-(5-chloro-6-(trifluoromethoxy)-1H-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (JNJ-42041935), a potent and selective hypoxia-inducible factor prolyl hydroxylase inhibitor. Mol Pharmacol. 2011;79:910–20.
Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200.
Leung CH, Chan DS, Ma VP, Ma DL. DNA-binding small molecules as inhibitors of transcription factors. Med Res Rev. 2013;33:823–46.
Hidaka T, Sugiyama H. Chemical approaches to the development of artificial transcription factors based on pyrrole-imidazole polyamides. Chem Rec. 2021;21:1374–84.
Liu J, Perumal NB, Oldfield CJ, Su EW, Uversky VN, Dunker AK. Intrinsic disorder in transcription factors. Biochemistry. 2006;45:6873–88.
Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, et al. Structure of a biologically active estrogen receptor-coactivator complex on DNA. Mol Cell. 2015;57:1047–58.
Yi P, Wang Z, Feng Q, Chou CK, Pintilie GD, Shen H, et al. Structural and functional impacts of ER coactivator sequential recruitment. Mol Cell. 2017;67:733–43 e734.
Yu X, Yi P, Hamilton RA, Shen H, Chen M, Foulds CE, et al. Structural insights of transcriptionally active, full-length androgen receptor coactivator complexes. Mol Cell. 2020;79:812–23 e814.
Callaway E. ‘It will change everything’: DeepMind’s AI makes gigantic leap in solving protein structures. Nature. 2020;588:203–4.
Cramer P. AlphaFold2 and the future of structural biology. Nat Struct Mol Biol. 2021;28:704–5.
Yang J, Anishchenko I, Park H, Peng Z, Ovchinnikov S, Baker D. Improved protein structure prediction using predicted interresidue orientations. Proc Natl Acad Sci USA. 2020;117:1496–503.
Schneider R, Maurin D, Communie G, Kragelj J, Hansen DF, Ruigrok RW, et al. Visualizing the molecular recognition trajectory of an intrinsically disordered protein using multinuclear relaxation dispersion NMR. J Am Chem Soc. 2015;137:1220–9.
Milles S, Salvi N, Blackledge M, Jensen MR. Characterization of intrinsically disordered proteins and their dynamic complexes: from in vitro to cell-like environments. Prog Nucl Magn Reson Spectrosc. 2018;109:79–100.
Henley MJ, Koehler AN. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat Rev Drug Discov. 2021;20:669–88.
Huynh K, Partch CL. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci. 2015;79:28.29.21–14.
Motoyaji T. Revolution of small molecule drug discovery by affinity selection-mass spectrometry technology. Chem Pharm Bull (Tokyo). 2020;68:191–3.
Olaru A, Bala C, Jaffrezic-Renault N, Aboul-Enein HY. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit Rev Anal Chem. 2015;45:97–105.
Uttamchandani M, Yao SQ. The expanding world of small molecule microarrays. Methods Mol Biol. 2017;1518:1–17.
Neri D, Lerner RA. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu Rev Biochem. 2018;87:479–502.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (22177063), the National Key R&D Program of China (2018YFE0113000), the Shandong Provincial Natural Science Foundation, China (ZR2021JQ30), and the Taishan Scholars Program of Shandong (tsqn201909004) to DLW, and grants from the National Natural Science Foundation of China (No. 92168120, 81974506, 81673486 and 81373405), and the Beijing Natural Science Foundation (No. Z200019 and 7172119) to LT.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhuang, Jj., Liu, Q., Wu, Dl. et al. Current strategies and progress for targeting the “undruggable” transcription factors. Acta Pharmacol Sin 43, 2474–2481 (2022). https://doi.org/10.1038/s41401-021-00852-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00852-9
Keywords
This article is cited by
-
Transcription factors in fibroblast plasticity and CAF heterogeneity
Journal of Experimental & Clinical Cancer Research (2023)
-
Hyperthermia promotes degradation of the acute promyelocytic leukemia driver oncoprotein ZBTB16/RARα
Acta Pharmacologica Sinica (2023)
-
Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials
Signal Transduction and Targeted Therapy (2023)