Abstract
Stroke is the major cause of death and disability worldwide. Most stroke patients who survive in the acute phase of ischemia display various extents of neurological deficits. In order to improve the prognosis of ischemic stroke, promoting endogenous neurogenesis has attracted great attention. Salvianolic acid A (SAA) has shown neuroprotective effects against ischemic diseases. In the present study, we investigated the neurogenesis effects of SAA in ischemic stroke rats, and explored the underlying mechanisms. An autologous thrombus stroke model was established by electrocoagulation. The rats were administered SAA (10 mg/kg, ig) or a positive drug edaravone (5 mg/kg, iv) once a day for 14 days. We showed that SAA administration significantly decreased infarction volume and vascular embolism, and ameliorated pathological injury in the hippocampus and striatum as well as the neurological deficits as compared with the model rats. Furthermore, we found that SAA administration significantly promoted neural stem/progenitor cells (NSPCs) proliferation, migration and differentiation into neurons, enhanced axonal regeneration and diminished neuronal apoptosis around the ipsilateral subventricular zone (SVZ), resulting in restored neural density and reconstructed neural circuits in the ischemic striatum. Moreover, we revealed that SAA-induced neurogenesis was associated to activating Wnt3a/GSK3β/β-catenin signaling pathway and downstream target genes in the hippocampus and striatum. Edaravone exerted equivalent inhibition on neuronal apoptosis in the SVZ, as SAA, but edaravone-induced neurogenesis was weaker than that of SAA. Taken together, our results demonstrate that long-term administration of SAA improves neurological function through enhancing endogenous neurogenesis and inhibiting neuronal apoptosis in ischemic stroke rats via activating Wnt3a/GSK3β/β-catenin signaling pathway. SAA may be a potential therapeutic drug to promote neurogenesis after stroke.
Similar content being viewed by others
Introduction
Stroke is the major cause of death and disability worldwide and the therapeutic drugs are limited. Thrombolysis is the mainly therapeutic strategy for acute stroke. However, due to its narrow treatment time window, most stroke patients cannot benefit from thrombolytic therapy and gain permanent vascular occlusion. Therefore, effective treatment for stroke continues to be an urgent clinical need. In order to reduce the rate of death and disability, and improve the prognosis of stroke, promoting neurorestoration after stroke has attracted more and more attention [1].
Neurorestoration includes neurogenesis, synaptic remodeling, and angiogenesis, of which neurogenesis has been observed in the adult human brain [2]. The dentate gyrus (DG) of the hippocampal and the subventricular zone (SVZ) of the lateral ventricles are considered neurogenic regions in the adult brain, containing neural stem/progenitor cells (NSPCs) [3]. Studies have demonstrated that neurogenesis occurs in the SVZ and DG in experimental stroke [4, 5]. Spontaneous neurogenesis in the SVZ after ischemic stroke is a dynamic continuum of events including the proliferation of NSPCs and migration to the ischemic region, differentiation into neurons with different phenotypes and functions, and functional integration into the neural network system. Inhibition of SVZ neurogenesis aggravates neurological deficits and hampers recovery after stroke [6], while neural precursor cell transplantation causes neurological improvement [7,8,9], suggesting that enhancement of endogenous neurogenesis is strongly related to the improvement of neurological function after stroke. Although the proliferation and migration of SVZ neuroblasts increase clearly after stroke, the survival rate of neuroblasts resided in the SVZ or migrating to the damaged tissue, and the degree of differentiation into neuron with different lineages are parlous low [10], leading to insufficient spontaneous neurogenesis in stroke patients to support brain repair and functional recovery. Therefore, strengthening ischemia-driven endogenous neurogenesis, promoting NSPCs proliferation, migration, and differentiation into mature neurons, and maintaining the survival of neurons in the SVZ may be a promising treatment for stroke.
Salvianolic acid A (SAA, Fig. 1a), the main water-soluble compound of the Salvia miltiorrhiza Bge. extract [11], has various pharmacological properties and is principally used for the treatment of cardiovascular and cerebrovascular diseases. Pharmacokinetic studies have revealed that the elimination half-life (T1/2) of SAA is 3.29 h [12], and after stroke, SAA is more preferentially distributed in the ischemic brain tissue [13], which may be the basis of the protective effect of SAA on cerebral ischemia. Currently, the researches of SAA on ischemic stroke mainly focus on its neuroprotective and preventive effects in the acute phase. The results suggest that SAA exerts anti-oxidative stress [14], anti-apoptosis [15,16,17], anti-inflammatory [17,18,19], and neurovascular protection [20, 21] effects to rescue damaged tissues. However, the roles of SAA on the long-term functional outcomes after stroke are still unknown [22]. Whether long-term administration of SAA affects neurological function by regulating endogenous neurogenesis post-stroke has not yet been studied. In the present study, the effects of SAA administrated for 14 d on the recovery of neurological function, endogenous neurogenesis, axonal regeneration, neuronal apoptosis after ischemic stroke, and its underlying mechanisms were investigated, which may provide a novel treatment strategy for improving the prognosis of stroke.
Materials and methods
Experimental animals
Male Sprague-Dawley rats (230–260 g, certificate No. SCXK (Jing) 2019–0008) were supplied by Beijing HFK Bioscience Co., Ltd. (Beijing, China). All animal procedures were carried out in line with the principles of the NIH Guide for the Care and Use of Laboratory Animals. And protocols were approved by the Institutional Animal Care and Use Committee of the Institute of Materia Medical, CAMS & PUMC. All methods were taken to minimize the number of rats used and their suffering.
Electrocoagulation-induced autologous thrombus stroke model
The autologous thrombus stroke model induced by electrocoagulation was established as previous study [23]. In short, chloral hydrate (4 mL/kg) was used to anesthetize rats. The right common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were bluntly separated. The CCA was fixed in the electric clamp of the YLS-14B thrombosis tester (Jinan Yiyan Science & Technology Development Co., Ltd., Jinan, China), and the galvanic stimulation (1.00 mA) was initiated for 5 min. The thrombus was shattered into homogeneous pieces, which were flushed into the ICA and middle cerebral artery. Then the CCA was clamped for another 15 min to stabilize the thrombus. Finally, the skin was sutured and the animal was returned to the cage. Except for electrocoagulation, the sham operation rats received all surgical procedures.
Experimental protocols and drug treatments
SAA (purity > 99% by HPLC, Fig. 1a) was prepared by the Institute of Materia Medica (Beijing, China). Edaravone injection, as the positive control, was obtained from Simcere Pharmaceutical Co., Ltd (H20031342, Nanjing, China). As shown in Fig. 1b, rats were randomly allocated into the sham operation group, model group, SAA (10 mg/kg, ig) + model group, and Edaravone (5 mg/kg, iv) + model group. Rats were administered 24 h after surgery, once a day for 14 consecutive days. Based on our previous study [24] and preliminary experiments (SAA 1, 3, 10 mg· kg−1· d−1, Supplementary Fig. S1), once daily administration of SAA 10 mg/kg was chosen in our current study. In order to evaluate the newborn cells, rats were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO, USA) (50 mg· kg−1 ·d−1) 24 h after surgery for 7 d. Behavioral testing and magnetic resonance imaging were implemented on 1, 7, and 14 d after surgery. At 14 d, the rats were sacrificed, and the samples were collected for further study.
Neurological function test
At 1, 7, and 14 d after surgery, neurological function tests were performed by an investigator blinded to the groups.
Longa scores and modified neurological severity scores (mNSS)
Zea-Longa scores were divided into five grades [25], higher scores denoted greater severity. According to mNSS, neurological functions, i.e., motor (6 points), sensory (2 points), reflexes (4 points) and balance (6 points), were graded on a numeric scale from 0 to 18 [26]. Rats with scores above or equal to 8 were used for further experiments.
Grasping ability test
Rats were individually suspended by forelimbs from a wire stretched horizontally. The grasping time of the rats and their behavior before landing were recorded and measured using a six-level scoring method (0–5) [20, 27], higher scores illustrated stronger muscular strength of the forelimbs.
Open field test
Rats were placed in the middle of a box for 5 min in a quiet environment. A video camera was used to record the movement route of each rat and the data were further analyzed by SuperMaze V2.0 (Shanghai XinRuan Information Technology Co., Ltd, Shanghai, China).
Magnetic resonance imaging (MRI)
MRI experiments were performed on Bruker PharmaScan 7.0 T/16 US system (Bruker Technology Co., Ltd, Karlsruhe, Germany). T2_TurboRARE, TR/TE = 3160/33, 3 Averages, 35 × 35 mm field of view, 0.5 mm layer thickness; TOF_3D_FLASH, TR/TE = 15/2.7, 2 Averages, 30 × 30 × 28 mm field of view. The infarct volume was corrected by using following formula for edema and atrophy: Cerebral infarct volume (%) = [infarct volume − (ipsilateral hemisphere volume − contralateral hemisphere volume)]/contralateral hemisphere volume × 100% [28].
Hematoxylin and eosin (H&E) staining
After transcardially perfused with 0.9% normal saline followed by 4% paraformaldehyde, the brains were isolated immediately and embedded into paraffin wax. Paraffin-embedded tissue was cut into 5 μm-thick sections. Then the sections were dewaxed, rehydrated, and stained with H&E.
Immunohistochemical staining
Coronal sections were dewaxed and rehydrated for immunohistochemical staining. Endogenous peroxidase activity was quenched by 3% H2O2 for 25 min, and nonspecific binding was also blocked by 3% BSA for 30 min. Sections were then incubated overnight at 4 °C with the primary antibodies (Table 1) and incubated with the corresponding secondary/fluorescent secondary antibodies (Table 1). Then, the immunohistochemical sections were co-incubated with 3,3′-diaminobenzidine peroxide (DAB) and counterstained with hematoxylin. The immunofluorescence sections were incubated with DAPI solution for 10 min at room temperature to counterstain the nuclei. Images were captured by the Nikon Eclipse Ti-SR (Nikon, Tokyo, Japan). The cumulative optical density, number of immunoreactive cells, and area of immunoreactive spots were measured by Image-Pro 10 software (Roper Industries, Inc. Sarasota, FL, USA). In this study, the SVZ and striatum of the corresponding sections were imaged, six rats per group and one section per rat were analyzed.
TUNEL staining assays
TUNEL Kit (Wuhan Servicebio Biotechnology Co., Ltd, Wuhan, China) was applied to test cell apoptosis in the SVZ. The cell apoptosis intensity was expressed as the number of TUNEL-positive cells and the ratio of apoptosis cells relative to total cells (DAPI-positive cells).
Western blot analysis
The brain tissue was lysed in RIPA buffer and centrifuged at 12,000 × g for 20 min at 4 °C. Equivalent amounts of protein were separated by SDS-PAGE and transferred for 2.5 h onto PVDF membranes. After blocking with 5% skim milk in TBST for 2 h, the membranes were incubated overnight at 4 °C with corresponding primary antibodies (Table 1). HRP-conjugated rabbit or mouse IgG secondary antibodies (Table 1) were then incubated for 2 h at room temperature. The antibody-bound bands were visualized using super ECL (Applygen Technologies Inc. Beijing, China) with the Tanon 4600 Imaging System (Tanon Technology Co., Ltd, Beijing, China). The band density was analyzed with ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All statistical analyses were performed by GraphPad Prism 7.00 software (GraphPad Software, San Diego, CA, USA), and all results were expressed as means ± SEM. The differences between the various groups were analyzed using one-way ANOVA with Tukey’s Multiple Comparison post hoc test. The mortality rate was analyzed with the Chi-square test. Neurological scores were performed using repeated measures two-way ANOVA followed by a Dunnett’s multiple comparison post hoc test. Statistical significance was considered at P < 0.05.
Results
SAA enhanced survival rate and facilitated the recovery of neurological function within 14 d after ischemia
As shown in Fig. 2a, ~34.0% of rats in the model group died within 3 d after ischemia and reached 42.55% at 14 d. Treatment with SAA showed significant improvement in survival rate (78.79% vs 57.45%). However, edaravone could not increase the survival rate. Within 3 d after ischemia, the body weight of rats decreased in the surgery group and then continued to increase over time, and SAA treatment group gained more weight compared with the model group (Fig. 2b). Four ethological studies were used to assess neurological function at different time points after ischemia. Compared with the sham group, the neurological function of the model group at each time point was significantly reduced on the mNSS scores (Fig. 2c), the Longa scores (Fig. 2d), the grasping ability test (Fig. 2e), and the open field test (Fig. 2f–h). Treatment with SAA or edaravone could improve the neurological deficits at each time point, and the effect was most significant at 14 d, which indicated that SAA promoted the recovery of neurological function as the ischemic time increased.
a Survival curves after stroke, n = 33–47. b Body weight. c The mNSS score. d Longa behavior test. e Prehensile ability test. f The movement routes. g Path length and (h) average velocity of rats in a box within 5 min. The data are presented as the mean ± SEM, n = 7–14; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs the sham group, *P < 0.05, **P < 0.01, and ***P < 0.001 vs the model group.
SAA alleviated ischemia induced brain injury
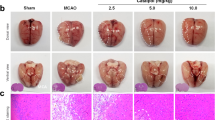
MRI was performed to assess infarct volume at 1, 7, 14 d and the vascular embolism at 14 d after ischemia. As shown in Fig. 3a, b, brain infarction was observed at different times after ischemia, and SAA significantly decreased the infarct volume at 7 d and 14 d, compared with the model group. Edaravone also showed a similar role at 14 d. Cerebrovascular imaging was performed by TOF_3D MRI to observe the vascular embolism. As shown in Fig. 3c, the ICA of the model group exhibited severe embolism and stenosis. This phenomenon was reversed by SAA treatment, which partially restored vascular patency and blood supply to the ischemic region. The neuron morphology in the striatum and hippocampus was detected by H&E staining. As shown in Fig. 3d, there were no apparent pathological changes occurred in the cerebrum tissue of sham rats. While in the model group, a large number of vacuole-like spaces appeared, the neuron structure was loose and atrophied, and the nucleus was pyknosis. SAA or edaravone treatment dramatically reversed this phenomenon, reduced the disruption of neurons, glial cells, and vascular elements, and restored the density of neurons.
a Representative images of DWI_T2 of brain. b Bar graph of infarct volume. c Representative images of TOF_3D of brain. d H&E staining of the hippocampal and striatum. The data are presented as the mean ± SEM, n = 6. ###P < 0.001 vs the sham group, *P < 0.05 and **P < 0.01 vs the model group. The images are at ×200 magnification.
SAA promoted NSPCs proliferation and migration around the SVZ at 14 d after ischemia
We further investigated the effects of SAA on NSPCs proliferation and migration at 14 d after ischemia. The numbers of Ki67 immunoreactive cells and Ki67/BrdU double immunoreactive cells in or near the ipsilateral SVZ in the model group were higher than that in the sham group (Fig. 4a–c). After SAA treatment, the numbers of Ki67 immunoreactive cells and Ki67/BrdU double immunoreactive cells significantly increased compared with model group. In addition, compared with the model group, the numbers of Nestin (an NSPCs marker) immunoreactive cells and Nestin/BrdU double immunoreactive cells were markedly raised in the SAA group (Fig. 4e–g). Besides, edaravone also increased the number of Ki67 immunoreactive cells, but had no significant effect on the proliferation of Nestin immunoreactive cells.
a Representative images of double-labeling immunostaining of Ki67/BrdU+ (Ki67-red and BrdU-green) in or near the SVZ. b The cumulative optical density of Ki67 in or near the SVZ. c The number of Ki67-BrdU double (+) cells in or near the SVZ. d The ratio of Ki67-BrdU double (+) cells among BrdU positive cells in or near the SVZ. e Representative images of double-labeling immunostaining of Nestin/BrdU+ (Nestin-red and BrdU-green) in or near the SVZ. f The cumulative optical density of Nestin in or near the SVZ. g The number of Nestin-BrdU double (+) cells in or near the SVZ. h The ratio of Nestin-BrdU double (+) cells among BrdU positive cells in or near the SVZ. The data are presented as the mean ± SEM, n = 6; #P < 0.05 and ##P < 0.01 vs the sham group, *P < 0.05 and **P < 0.01 vs the model group. The images are at ×200 magnification.
To investigate the migratory and wide distribution capability of NSPCs to the ischemic region, we measured the farthest migration distance of DCX-immunoreactive cells (neuroblasts) from the SVZ to the striatum. The migration distance of neuroblasts in the SAA treatment group was longer than that of the model group (414.50 ± 37.99 μm vs 169.70 ± 35.32 μm) (Fig. 5e, i). Edaravone also performed similar effect on the migration of NSPCs. These results indicated that SAA administration for 14 d enhanced the proliferation of NSPCs and promoted the migration of neuroblasts after ischemia.
a Representative images of double-labeling immunostaining of MAP2/BrdU+ (MAP2-red and BrdU-green) in the striatum. b The cumulative optical density of MAP2 in the striatum. c The number of MAP2-BrdU double (+) cells in the striatum. d The ratio of MAP2-BrdU double (+) cells among BrdU positive cells in the striatum. e Representative images of double-labeling immunostaining of DCX/BrdU+ (DCX-red and BrdU-green) in or near the SVZ. f The cumulative optical density of DCX in or near the SVZ. g The number of DCX-BrdU double (+) cells in or near the SVZ. h The ratio of DCX-BrdU double (+) cells among BrdU positive cells in or near the SVZ. i The longest distance between the location of DCX ( + ) cells and the SVZ. j–m Representative images of MAP2 and GAP43 expression in the striatum and hippocampus. n Representative images of immunohistochemical staining and the pixel area of GAP43 in the striatum. o Bar graph of optical stain intensity from all groups. The data are presented as the mean ± SEM, n = 6; #P < 0.05, ##P < 0.01, and ####P < 0.0001 vs the sham group, *P < 0.05, **P < 0.01 and ***P < 0.001 vs the model group. The images are at ×100 or 200 magnification.
SAA promoted NSPCs differentiation into neurons and axonal regeneration at 14 d after ischemia
Ischemia caused substantial neuron loss in the model group, the number of MAP2 positive cells (mature neurons) in the striatum was obviously lower than the sham group (Fig. 5a, b). Western blot further confirmed that the expression of MAP2 protein in the striatum and hippocampus in the model group was significantly decreased (Fig. 5j, l). SAA or edaravone treatment for 14 d remarkably increased the density of mature neurons in the striatum, and promoted the expression of MAP2 protein both in the striatum and hippocampus.
To explore the mechanisms of the increased mature neurons after SAA treatment, the effect of SAA on the differentiation of NSPCs into neurons was measured. Either in or near the SVZ, SAA dramatically promoted the numbers of DCX immunoreactive cells and DCX/BrdU double immunoreactive cells (Fig. 5e–g). Furthermore, the number of MAP2/BrdU double positive cells in the striatum after SAA treatment was higher than that in the model group (Fig. 5c). In addition, among BrdU immunoreactive cells, the ratios of Ki67, Nestin, DCX, and MAP2 immunoreactive cells were evidently higher after SAA treatment than that in the model group, respectively (Figs. 4d, h, 5d, h). But no significant promotion effect was observed in the edaravone group. Besides, the newborn neuroblasts in the SVZ also migrated to the peri-infarct cortex and differentiated into mature neurons. SAA increased the density of mature neurons in the peri-infarct cortex (Supplementary Fig. S2). These results demonstrated that SAA contributed to the increment of mature neuron density 14 d after ischemia, which may be associated with the differentiation of NSPCs into neurons.
Functional recovery and neurogenesis after stroke are mediated by the reestablishment of axonal connections between neurons in the ischemic region [29]. GAP43 is an important marker of axonal sprouting. As shown in Fig. 5k and m, the results of Western blot showed that the expression of GAP43 was obviously decreased in the model rats compared to the sham rats, and this decrease was reversed by SAA treatment in the hippocampus and striatum. Immunohistochemistry assays further proved that treatment with SAA appreciably increased positive staining of GAP43 in the striatum (Fig. 5n, o). Besides, edaravone also promoted the expression and positive staining of GAP43. Taken together, these results indicated that SAA promoted long-term functional recovery by enhancing the proliferation, migration, differentiation of NSPCs, and reestablishing inter-neuronal communication.
SAA promoted endogenous neurogenesis through regulating Wnt3a/GSK3β/β-catenin signaling pathway at 14 d after ischemia
To further explore the underlying mechanism of SAA promoting endogenous neurogenesis, Wnt3a/GSK3β/β-catenin signaling pathway was investigated by immunofluorescence and Western blot, which was involved in cell growth and proliferation, especially neuron survival. The results of immunofluorescence assay showed that compared with the model group, treatment with SAA appreciably increased the expression of Wnt3a and β-catenin in the SVZ (Fig. 6a–c). Consistently, Western blot further confirmed that during ischemic stroke, the ratio of p-GSK3β/GSK3β was notably decreased, indicating that the GSK3β/β-catenin pathway was inhibited. Treatment with SAA markedly up-regulated the expression of Wnt3a (an upstream signal for GSK3β) in the striatum and hippocampus, which was reduced after ischemia, leading to phosphorylation and inactivation of GSK3β. Accordingly, the ratio of p-GSK3β/GSK3β and the expression of β-catenin were elevated, indicating that SAA promoted the activation of Wnt3a/GSK3β/β-catenin signaling pathway (Fig. 6d–g). Moreover, the expression of TCF-4 (a downstream transcriptional factor of β-catenin), which promotes the expression of Wnt pathway target genes, was dramatically increased in the SAA-treated group in the striatum and hippocampus compared to the model group (Fig. 6h, i). Meanwhile, with the activation of β-catenin and TCF-4, the levels of CyclinD1, NeuroD1, and BDNF were also increased notably in the SAA-treated group (Fig. 6h, j–l). In addition, edaravone could also activate Wnt3a/GSK3β/β-catenin signaling pathway by regulating the expression of the above proteins. These observations revealed that long-term administration of SAA promoted neurogenesis by facilitating the expression of Wnt3a to activate the GSK3β/β-catenin signaling pathway after ischemic stroke.
a Representative images of expression of Wnt3a and β-catenin in the SVZ. b and c The cumulative optical density of Wnt3a and β-catenin in the SVZ. d–l Representative images of Wnt3a, p-GSK3β, GSK3β, β-catenin, TCF-4, CyclinD1, NeuroD1and BDNF expression in the striatum and hippocampus. The data are presented as the mean ± SEM, n = 3 or 6. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs the sham group, *P < 0.05, **P < 0.01 and ***P < 0.001 vs the model group.
SAA inhibited neuronal apoptosis in the SVZ by regulating the expression of apoptosis-related proteins at 14 d after ischemia
Inhibiting the apoptosis of immature and mature neurons in the SVZ further supports neurogenesis and promotes the recovery of neurological function after ischemia. After 14 d of ischemia, the TUNEL staining in the SVZ showed distinctively enhancement in the number and ratio of apoptotic cells, which were weakened by SAA treatment (Fig. 7a–c). In addition, the expression of apoptosis-related proteins in the hippocampus and striatum was measured by Western blot. Compared with the model group, SAA apparently up-regulated p-CREB, another downstream protein of GSK3β/β-catenin, accompanied by the obvious increase of the Bcl-2/Bax ratio (Fig. 7d–f). Furthermore, the decreased expression of cleaved caspase 3 and cleaved caspase 9 (Fig. 7g–i) in the SAA group indicated the inhibition of neuronal apoptosis. Edaravone could also improve the expression of P-CREB and Bcl-2/Bax ratio to inhibit neuronal apoptosis. These results further indicated that SAA inhibited neuronal apoptosis and promoted cell survival in the SVZ by enhancing the expression levels of CREB/Bcl-2 at 14 d after ischemia, which may be associated with its neurogenesis effects.
a Representative images of TUNEL staining in the SVZ of stroke rats. b The number of TUNEL immunoreactive cells in the SVZ. c The ratio of apoptosis cells relative to total cells in the SVZ. d–i Representative images of p-CREB, CREB, Bcl-2, Bax, cleaved caspase-3 and cleaved caspase-9 expression in the striatum and hippocampus. The data are presented as the mean ± SEM, n = 6. #P < 0.05, ##P < 0.01 and ###P < 0.001 vs the sham group, *P < 0.05, **P < 0.01 and ***P < 0.001 vs the model group. The images are at ×100 magnification.
Discussion
Current clinical therapeutical strategies for stroke include thrombolysis, treatment of stroke-related complications, and prevention of recurrence [30]. Most stroke patients who survive in the acute phase of ischemia experience various neurological deficits [31]. Therefore, it is of great significance to promote long-term recovery of neurological function and improve the patients’ quality of life after ischemic stroke. In the present study, we firstly demonstrated that long-term administration of SAA could promote the recovery of neurological function after stroke by enhancing the NSPCs proliferation in the SVZ, migration to the ischemic striatum, and differentiation into neurons, reestablishing inter-neuronal communication, and inhibiting neuronal apoptosis, which may be associated with the activation of the Wnt3a/β-catenin signaling pathway.
In the study, a new electrocoagulation-induced autologous thrombus stroke rat model was used to assess the neural repair effect of SAA. Currently, the ideal animal models for studying neural repair after stroke are limited [32]. The autologous thrombus stroke model used in this study is different from the traditional embolic stroke model [19], the formation and development of thrombosis and infarction in this model are more similar to that of human ischemic stroke [22, 33], and the reproducible and predictable infarct volume is also provided in this model, which can better evaluate the role of SAA in facilitating neurogenesis based on improving thromboembolism.
The subsequent massive loss of neurons after ischemic stroke leads to sensorimotor and cognitive deficits, and 70%–80% of surviving patients develop hemiplegia [34]. Therefore, the prime goal of stroke treatment is to alleviate ischemic injury and restore neurological function. In previous studies, we have shown that SAA alleviated neurological deficits in ischemic stroke rats at 24 h after ischemia [17, 19], indicating the potential of SAA in the recovery of neurological function in the acute phase of stroke. In this study, we also found that administration with SAA promoted the recovery of neurological function as the ischemic time increased, including the ability of memory and learning spatial, motor function. Interestingly, we also observed that SAA ameliorated the thromboembolism, promoted vascular recanalization, and decreased the infarct volume, partially regained the blood and nutrient supply in the ischemic site, which may be the basis for SAA to promote neurogenesis and axon remodeling [35].
At present, neurorestoration therapy for stroke is gaining more attention, and it has become a novel strategy to improve the prognosis of ischemic stroke [36, 37]. Since exogenous stem cell transplantation may activate the immune system and induce tumors [38], promoting endogenous neurogenesis has become the mainstay for neurorestoration therapy [36, 37]. Activation of endogenous neurogenesis, including promoting NSPCs directional proliferation, migration and differentiation into neurons, can facilitate the recovery of ischemic brain structure and function through the accurate replacement of neurons and the reconstruction of neural circuits [39, 40]. The proliferation and differentiation of NSPCs in the SVZ and DG are both strongly stimulated after cerebral ischemia [41, 42]. The SVZ newborn neuroblasts migrate to the infarct boundaries of the cortex and striatum, and differentiate into mature neurons to replace the lost neurons. However, newborn neurons in the DG have no ability to migrate toward the ischemic striatum and cortex. Neurogenesis and newborn neuron integration are confined to the granular cell layer [3], which helps maintain the hippocampal function. Considering that the hippocampus is the paramount adult brain region for the formation of cognitive functions and spatial memories [43], it is still of great significance in the detection of related marker proteins and pathways in this study. Therefore, the effect of SAA on the neurogenesis in the DG after cerebral ischemia will be further explored.
Neurogenesis by cell cycle-related BrdU labeling determines the proliferation, migration, and differentiation of NSPCs after ischemic stroke. However, BrdU can mark the division process of irrelevant cells, such as the DNA repair after mitosis and apoptosis process [44]. Ki67 is expressed during the mitotic cell cycle, and the detection is not interfered by the DNA repair process [45]. Therefore, we performed dual immunofluorescence with BrdU and Ki67. Our results indicated that SAA increased the number of Ki67/BrdU and Nestin/BrdU double immunoreactive cells in the SVZ and surrounding area, indicating that SAA enhanced the proliferation of endogenous NSPCs. To participate in repair, newborn neurons must be able to reach the ischemic region. SAA treatment strengthened the migratory capacity of NSPCs, accompanied by a longer migration distance of DCX immunoreactive cells from the SVZ to the ischemic striatum. Determining differentiation and its phenotype is a crucial step for neurogenesis. DCX/BrdU and MAP2/BrdU dual-labeling were used to determine the phenotype of newborn cells. SAA treatment stimulated NSPCs to differentiate into mature neurons phenotype and integrate into existing neuronal circuits in the striatum. Interestingly, 14 d after cerebral ischemia, NSPCs in the model rats showed stronger proliferation, migration, and differentiation capabilities, but the density of mature neurons was still dramatically lower than that in the sham rats, illustrating that stroke gave rise to compensatory enhancement of neurogenesis, but still insufficient for brain repair. SAA administration for 14 d clearly enhanced endogenous neurogenesis. This is firstly reported that SAA could promote the NSPCs proliferation, migration, and differentiation into neurons after stroke.
Rapid and dramatic loss of axons and delayed axonal degeneration occur with the ischemic time. The loss of axons after stroke highlights the vital role of axonal regeneration in neurogenesis and functional recovery [46]. Our previous study has been shown that SAA promoted the regeneration of myelin in diabetic peripheral neuropathy [24]. However, the role of SAA on axonal regeneration after stroke is still unknown. In the present study, our results suggested that SAA treatment for 14 d spurred axonal sprouting, and reestablished new axonal connections in the ischemic striatum, which contributed to the recovery of neurological function after stroke.
In adult mammal ischemic stroke, neurogenesis involves multiple signaling pathways, among which the canonical Wnt/GSK3β/β-catenin pathway is of great importance in neurogenesis [47]. Various therapeutic strategies are explored to target endogenous neurogenesis regulated by Wnt/GSK3β/β-catenin signaling pathway after stroke [48,49,50]. The Wnt pathway is a highly conserved signal transduction pathway, which promotes and maintains development [51] and regulates pivotal biological processes, such as cell proliferation, migration, and fate determination during development [52, 53]. Wnt3a, as a key molecule of the Wnt family, is a primary regulator of adult NSPCs proliferation and differentiation [54, 55]. Wnt3a ligand interacts with Wnt-Frizzled mediated by the low-density lipoprotein receptor-related protein-5/6 (LRP5/6) co-receptor, leading to phosphorylation and inactivation of GSK3β. Previous studies have proved that the inhibition of GSK3β is also a potential target for promoting neurogenesis after ischemic stroke [56]. β-catenin dissociates from Axin/APC/GSK3β complex, accumulates in the cytoplasm and translocates to the nucleus. In the nucleus, β-catenin stimulates the expression of target genes through interaction with TCF/LEF, including CREB, BDNF, CyclinD1 [57] and NeuroD1 [58], which facilitates NSPCs proliferation and differentiation, and maintains the survival of newborn neurons. In this study, we confirmed that Wnt3a/GSK3β/β-catenin signaling pathway was inhibited at 14 d after ischemic injury, which was consistent with the previous study [59]. However, SAA suppressed GSK3β activity through the up-regulation of Wnt3a, thereby promoting the accumulation of β-catenin and the expression of downstream proteins in the SVZ, striatum, and hippocampus. Taken together, SAA promoted multiple aspects of endogenous neurogenesis by activating Wnt3a/GSK3β/β-catenin pathways (Fig. 8).
After stroke, the Wnt3a/GSK3β/β-catenin signaling pathway is inhibited, leading to neuronal apoptosis and neurological deficits. Treatment with SAA promotes the expression of Wnt3a ligand, leading to the Wnt3a-dependent inhibition of GSK3β. β-catenin accumulates in the cytoplasm and translocates to the nucleus, interacts with TCF/LEF and stimulates target genes expression, including CREB, BDNF, CyclinD1 and NeuroD1, facilitating the proliferation, migration and differentiation of NSPCs. In addition, SAA increases the ratio of Bcl-2/Bax and inhibits the expression of caspase-3 and casepase-9 through the enhancement of CREB, which promotes the survival of immature and mature neurons. SAA improves the neurological function through the enhancement of neurogenesis and inhibition of neuronal apoptosis via Wnt3a/GSK3β/β-catenin signaling pathway after ischemic stroke.
As discussed earlier, the survival of neuroblasts that are newborn and migrated to the ischemic regions can be hampered, with ~80% of newborn neurons dying within 2 weeks [60]. Increased TUNEL activity was found in the SVZ after 9 d of stroke [61], indicating that apoptosis was related to the death of neuroblasts. In addition, the Wnt signaling pathway has been proved to stimulate the apoptosis-related CREB/Bcl-2 signaling cascade. Bcl-2 promotes the survival of immature and mature neurons in the cortex, hippocampus, and striatum after ischemia [62]. Chien et al. have shown that SAA can alleviate ischemic brain injury through enhancing Bcl-2 mediated anti-apoptosis at 1 d after cerebral ischemia [19]. However, the effect of SAA on anti-apoptosis to rescue the immature and mature neurons in the SVZ during the recovery phase of stroke is unclear. In this study, we found that after 14 d of stroke, the TUNEL activity in the SVZ was significantly enhanced, suggesting that apoptosis still played a crucial role in the recovery period of stroke. SAA administration for 14 d inhibited immature and mature neuronal apoptosis and promoted cell survival in the SVZ by activating CREB mediated up-regulation of the Bcl-2/Bax ratio and inhibiting the expression of caspase 3 and caspase 9. Therefore, our results supported that SAA may further improve neurogenesis by promoting the survival of the endogenous neuroblasts in the recovery period of stroke, resulting in more newborn neurons and better functional recovery.
As a free-radical scavenger, edaravone is one of the most routinely used brain protectants in the clinic, which is used to improve neurological deficits caused by acute ischemic stroke. Studies have found that edaravone scavenges free radicals [63], inhibits the inflammatory process, regulates the levels of matrix metalloproteinases and nitric oxide [64,65,66], promotes the proliferation and differentiation of NSPCs in the DG [67,68,69]. In the present study, 14 d after intravenous injection of edaravone, it partially promoted the proliferation, migration, differentiation of NSPCs, and axonal sprouting, which was weaker than that of SAA treatment. However, edaravone significantly inhibited neuronal apoptosis in the SVZ, which was equivalent to SAA. The difference between edaravone and SAA may be related to the markedly inhibitory effect of edaravone on endogenous reactive oxygen species [67, 70, 71].
In summary, compared with the previous studies on the treatment and prevention of SAA in the acute phase of ischemic stroke, this study firstly explored the effect of long-term administration of SAA on neurological function during the recovery period after stroke and its underlying mechanism related to neurogenesis. These findings may contribute to expanding the clinical indications of SAA and providing a novel therapeutical strategy for stroke.
Conclusions
Our findings demonstrated that long-term administration of SAA accelerated neurological recovery by promoting endogenous neurogenesis and axonal sprouting, and inhibiting neuronal apoptosis after ischemic stroke via Wnt3a/β-catenin signaling pathway. Therefore, SAA may be a potential therapeutic drug to promote neurogenesis after stroke, which can help improve the long-term prognosis of stroke.
References
Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6:238–44.
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–202.
Abbott LC, Nigussie F. Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol. 2020;49:3–16.
Tanaka R, Yamashiro K, Mochizuki H, Cho N, Onodera M, Mizuno Y, et al. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke. 2004;35:1454–9.
Yamashita T, Ninomiya M, Hernández Acosta P, García-Verdugo JM, Sunabori T, Sakaguchi M, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36.
Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:7993–8.
Bacigaluppi M, Pluchino S, Peruzzotti JL, Jametti LP, Kilic E, Kilic U, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–51.
Yoshida Y, Takagi T, Kuramoto Y, Tatebayashi K, Shirakawa M, Yamahara K, et al. Intravenous administration of human amniotic mesenchymal stem cells in the subacute phase of cerebral infarction in a mouse model ameliorates neurological disturbance by suppressing blood brain barrier disruption and apoptosis via immunomodulation. Cell Transplant. 2021;30:9636897211024183.
Asgari Taei A, Nasoohi S, Hassanzadeh G, Kadivar M, Dargahi L, Farahmandfar M. Enhancement of angiogenesis and neurogenesis by intracerebroventricular injection of secretome from human embryonic stem cell-derived mesenchymal stem cells in ischemic stroke model. Biomed Pharmacother. 2021;140:111709.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70.
Lian-Niang L, Rui T, Wei-Ming C. Salvianolic Acid A, a New Depside from Roots of Salvia miltiorrhiza. Planta Med. 1984;50:227–8.
Pei LX, Bao YW, Wang HD, Yang F, Xu B, Wang SB, et al. A sensitive method for determination of salvianolic acid A in rat plasma using liquid chromatography/tandem mass spectrometry. Biomed Chromatogr. 2008;22:786–94.
Feng SQ, Aa N, Geng JL, Huang JQ, Sun RB, Ge C, et al. Pharmacokinetic and metabolomic analyses of the neuroprotective effects of salvianolic acid A in a rat ischemic stroke model. Acta Pharmacol Sin. 2017;38:1435–44.
Zhang W, Song J-K, Yan R, He G-R, Zhang X, Zhou Q-M, et al. Salvianolic acid A alleviate the brain damage in rats after cerebral ischemia-reperfusion through Nrf2/HO-1 pathway. Yao Xue Xue Bao. 2016;51:1717–23.
Qian W, Wang Z, Xu T, Li D. Anti-apoptotic effects and mechanisms of salvianolic acid A on cardiomyocytes in ischemia-reperfusion injury. Histol Histopathol. 2019;34:223–31.
Song J, Zhang W, Wang J, Yang H, Zhou Q, Wang H, et al. Inhibition of FOXO3a/BIM signaling pathway contributes to the protective effect of salvianolic acid A against cerebral ischemia/reperfusion injury. Acta Pharm Sin B. 2019;9:505–15.
Zhao J, Li L, Fang G. Salvianolic acid A attenuates cerebral ischemia/reperfusion injury induced rat brain damage, inflammation and apoptosis by regulating miR-499a/DDK1. Am J Transl Res. 2020;12:3288–301.
Zhang W, Song JK, Zhang X, Zhou QM, He GR, Xu XN, et al. Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin J Nat Med. 2018;16:184–93.
Chien MY, Chuang CH, Chern CM, Liou KT, Liu DZ, Hou YC, et al. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic Biol Med. 2016;99:508–19.
Liu CD, Liu NN, Zhang S, Ma GD, Yang HG, Kong LL, et al. Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway. Acta Pharmacol Sin. 2021;42:370–81.
Mahmood Q, Wang GF, Wu G, Wang H, Zhou CX, Yang HY, et al. Salvianolic acid A inhibits calpain activation and eNOS uncoupling during focal cerebral ischemia in mice. Phytomedicine. 2017;15:8–14.
Jiao CX, Zhou H, Yang CX, Ma C, Yang YX, Mao RR, et al. Protective efficacy of a single salvianolic acid A treatment on photothrombosis-induced sustained spatial memory impairments. Neuropsychiatr Dis Treat. 2017;26:1181–92.
Ma YZ, Li L, Song JK, Niu ZR, Liu HF, Zhou XS, et al. A novel embolic middle cerebral artery occlusion model induced by thrombus formed in common carotid artery in rat. J Neurol Sci. 2015;359:275–9.
Xu C, Hou B, He P, Ma P, Yang X, Yang X, et al. Neuroprotective effect of salvianolic acid A against diabetic peripheral neuropathy through modulation of Nrf2. Oxid Med Cell Longev. 2020;2020:6431459.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8.
Bárez-López S, Bosch-García D, Gómez-Andrés D, Pulido-Valdeolivas I, Montero-Pedrazuela A, Obregon MJ, et al. Abnormal motor phenotype at adult stages in mice lacking type 2 deiodinase. PLoS ONE. 2014;9:e103857.
Kong LL, Wang ZY, Han N, Zhuang XM, Wang ZZ, Li H, et al. Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. Neuroinflammation. 2014;11:112.
Hinman JD. The back and forth of axonal injury and repair after stroke. Curr Opin Neurol. 2014;27:615–23.
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47.
Li Y, Huang J, He X, Tang G, Tang Y-H, Liu Y, et al. Postacute stromal cell-derived factor-1α expression promotes neurovascular recovery in ischemic mice. Stroke. 2014;45:1822–9.
Wang SN, Wang Z, Xu TY, Cheng MH, Li WL, Miao CY. Cerebral Organoids Repair Ischemic Stroke Brain Injury. Transl Stroke Res. 2020;11:983–1000.
Ma Y, Li L, Niu Z, Song J, Lin Y, Zhang H, et al. Effect of recombinant plasminogen activator timing on thrombolysis in a novel rat embolic stroke model. Pharmacol Res. 2016;107:291–9.
Patel RaG, Mcmullen PW. Neuroprotection in the Treatment of Acute Ischemic Stroke. Prog Cardiovasc Dis. 2017;59:542–8.
Ruan L, Wang B, Zhuge Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–73.
George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87:297–309.
Janowski M, Wagner DC, Boltze J. Stem cell-based tissue replacement after stroke: factual necessity or notorious fiction? Stroke. 2015;46:2354–63.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45.
Song P, Xia X, Han T, Fang H, Wang Y, Dong F, et al. BMSCs promote the differentiation of NSCs into oligodendrocytes via mediating Id2 and Olig expression through BMP/Smad signaling pathway. Biosci Rep. 2018;38:BSR20180303.
Daynac M, Morizur L, Chicheportiche A, Mouthon M-A, Boussin FD. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci Rep. 2016;6:21505.
Cuartero MI, De La Parra J, Pérez-Ruiz A, Bravo-Ferrer I, Durán-Laforet V, García-Culebras A, et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J Clin Invest. 2019;129:1536–50.
Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–8.
Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914.
Von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–20.
Von Bohlen Und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345:1–19.
Li MZ, Zhang Y, Zou HY, Ouyang JY, Zhan Y, Yang L, et al. Investigation of Ginkgo biloba extract (EGb 761) promotes neurovascular restoration and axonal remodeling after embolic stroke in rat using magnetic resonance imaging and histopathological analysis. Biomed Pharmacother. 2018;103:989–1001.
Xu D, Li F, Xue G, Hou K, Fang W, Li Y. Effect of Wnt signaling pathway on neurogenesis after cerebral ischemia and its therapeutic potential. Brain Res Bull. 2020;164:1–13.
Xu D, Hou K, Li F, Chen S, Fang W, Li Y. XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/beta-catenin signaling dependent way. Life Sci. 2019;235:116844.
Yang X, Song D, Chen L, Xiao H, Ma X, Jiang Q, et al. Curcumin promotes neurogenesis of hippocampal dentate gyrus via Wnt/beta-catenin signal pathway following cerebral ischemia in mice. Brain Res. 2021;1751:147197.
Sun FL, Wang W, Zuo W, Xue JL, Xu JD, Ai HX, et al. Promoting neurogenesis via Wnt/beta-catenin signaling pathway accounts for the neurorestorative effects of morroniside against cerebral ischemia injury. Eur J Pharmacol. 2014;738:214–21.
Van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–14.
Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864.
Kriska J, Janeckova L, Kirdajova D, Honsa P, Knotek T, Dzamba D, et al. Wnt/β-catenin signaling promotes differentiation of ischemia-activated adult neural stem/progenitor cells to neuronal precursors. Front Neurosci. 2021;15:628983.
Wiese KE, Nusse R, Van Amerongen R. Wnt signalling: conquering complexity. Development. 2018;145:dev165902.
Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5.
Chern CM, Wang YH, Liou KT, Hou YC, Chen CC, Shen YC. 2-Methox-ystypandrone ameliorates brain function through preserving BBB integrity and promoting neurogenesis in mice with acute ischemic stroke. Biochem Pharmacol. 2014;87:502–14.
Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7.
Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–105.
Menet R, Lecordier S, Elali A. Wnt pathway: an emerging player in vascular and traumatic mediated brain injuries. Front Physiol. 2020;11:565667.
Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–9.
Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, et al. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–30.
Lei ZN, Liu F, Zhang LM, Huang YL, Sun FY. Bcl-2 increases stroke-induced striatal neurogenesis in adult brains by inhibiting BMP-4 function via activation of β-catenin signaling. Neurochem Int. 2012;61:34–42.
Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–2.
Kikuchi K, Uchikado H, Miyagi N, Morimoto Y, Ito T, Tancharoen S, et al. Beyond neurological disease: new targets for edaravone (Review). Int J Mol Med. 2011;28:899–906.
Yamashita T, Shoge M, Oda E, Yamamoto Y, Giddings JC, Kashiwagi S, et al. The free-radical scavenger, edaravone, augments NO release from vascular cells and platelets after laser-induced, acute endothelial injury in vivo. Platelets. 2006;17:201–6.
Wang JH, Qu Y, Ray PS, Guo H, Huang J, Shin-Sim M, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J Clin Invest. 2011;121:212–25.
Kikuta M, Shiba T, Yoneyama M, Kawada K, Yamaguchi T, Hinoi E, et al. In vivo and in vitro treatment with edaravone promotes proliferation of neural progenitor cells generated following neuronal loss in the mouse dentate gyrus. Pharmacol Sci. 2013;121:74–83.
Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int. 2010;56:740–6.
Yoneyama M, Kawada K, Shiba T, Ogita K. Endogenous nitric oxide generation linked to ryanodine receptors activates cyclic GMP/protein kinase G pathway for cell proliferation of neural stem/progenitor cells derived from embryonic hippocampus. J Pharm Sci. 2011;115:182–95.
He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–53.
Zucker B, Hanusch J, Bauer G. Glutathione depletion in fibroblasts is the basis for apoptosis-induction by endogenous reactive oxygen species. Cell Death Differ. 1997;4:388–95.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 82004071); Beijing Municipal Natural Science Foundation (Grant No. 7182113); Fundamental Research Funds for the Central Universities (Grant No. 3332020038).
Author information
Authors and Affiliations
Contributions
SZ, GHD, and LLK conceptualized the study. SZ, DWK, GDM, YJY, SL, and ZRP contributed to the animal experiments. SZ, CDL, NJ, and WZ contributed to the molecular biology experiments. SZ contributed to the data collection and statistical analysis. SZ, GHD, and LLK drafted and finalized the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, S., Kong, Dw., Ma, Gd. et al. Long-term administration of salvianolic acid A promotes endogenous neurogenesis in ischemic stroke rats through activating Wnt3a/GSK3β/β-catenin signaling pathway. Acta Pharmacol Sin 43, 2212–2225 (2022). https://doi.org/10.1038/s41401-021-00844-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00844-9
Keywords
This article is cited by
-
Chronic vascular pathogenesis results in the reduced serum Metrnl levels in ischemic stroke patients
Acta Pharmacologica Sinica (2024)