Abstract
Brexpiprazole (Bre) is a new multi-target antipsychotic drug (APD) approved by the US FDA in 2015, and shows good therapeutic potential. But it lacks assessments on the metabolic side effects, which obstructs the treatment of schizophrenia. Glucagon-like peptide 1 (GLP1), an incretin associated with insulin action and metabolism, is involved in the metabolic syndrome (MS) caused by most APDs. In this study, we examined the adverse effects of Bre on glycolipid metabolism in rats and determined whether GLP1 was involved in Bre-caused MS. In the first part of experiments, rats were orally administered Bre (0.5 mg· kg−1· d−1) for 28 days with aripiprazole (1.0 mg· kg−1· d−1) or olanzapine (1.0 mg· kg−1· d−1) as the controls. Compared to vehicle, Bre administration significantly increased the weight gain, serum lipid (TG, TC, LDL, FFA), and blood glucose levels accompanied by the hormonal (insulin, glucagon, GLP1) imbalance, and the impaired glucose tolerance and insulin sensitivity. Moreover, we demonstrated that Bre administration significantly decreased the protein and mRNA levels of GLP1 in pancreas and small intestine by suppressing CaMKIIα, AMPK, and β-catenin; Bre administration also caused islet dysfunction with decreased GLP1R, PI3K, IRβ expression in pancreas, and the interference of IRS1, PI3K, p-AKT, and GLUT4 expression in the liver and skeletal muscle that represented the insulin resistance. In the second part of experiments, rats were orally administered Bre (0.5 mg· kg−1· d−1) for 42 days. We showed that co-administration with the GLP1 receptor (GLP1R) agonist liraglutide (0.125 mg· kg−1· d−1, ip) could ameliorate Bre-caused metabolic abnormalities. Our results demonstrate that GLP1/GLP1R signaling is involved in Bre-induced glycolipid metabolic disorders and co-treatment with liraglutide is an effective intervention against those abnormal metabolisms.
Similar content being viewed by others
Introduction
Schizophrenia is a relatively common chronic psychiatric disorder, with a life expectancy of 14–20 years shorter than that of the general population and 35% unnatural deaths attributed to cardiovascular disease and diabetes [1,2,3]. Although schizophrenia itself has relationships with an increased risk of developing metabolic syndrome (MS) and diabetes [4], it is also a consensus that antipsychotic drugs (APDs) can ulteriorly aggravate the metabolic defects. A growing number of evidences showed that APDs could significantly increase the incidence of weight gain and diabetes, especially olanzapine (Ola) with the most significant risk while aripiprazole (Ari) with a lower risk [5,6,7]. Drug-induced metabolic disorders are one of the major problems associated with the treatment of psychiatric disorders, leading to noncompliance and cessation of an otherwise efficient pharmacological treatment in some patients [8]. It is undoubtedly a huge threat to patients, and the treatment plans need to be improved based on the mechanism of APDs side effects.
Actually, APDs with high efficacy and low side effects are still under development. Brexpiprazole (Rexultid, Bre), a new atypical APD developed under the promotion of limited efficacy and multiple adverse drug reactions of current APDs, was approved by the USA FDA in 2015 [9]. Multiple clinical research results showed that Bre could improve the positive and negative syndrome scale (PANSS) score of adult patients with acute schizophrenia. One of the studies (NCT02054702 studies) [10] reported that Bre or Ari can improve the patients’ total score of PANSS and specific function levels while Bre was superior to Ari in Barratt Impulsiveness Scale. These findings indicate that Bre has good potential and advantages in the treatment of schizophrenia. However, it still lacks a full assessment of long-term safety and tolerability. Whether Bre, as the latest generation of APDs, has improved metabolic effects is worthy of further discussion. Therefore, in this study, we analyze the effect of Bre on metabolism in rats using its structural analog Ari as a control, under the premise that Ola was used as a positive control to confirm the successful establishment of a drug-induced MS rat model.
Our research focuses on the adverse effects of Bre on glycolipid metabolism, because diabetes is an important vital cause of death in schizophrenia patients. Generally, type-2 diabetes is characterized by obesity, insulin resistance (IR), and β-cell dysfunction, therefore the pathological mechanisms of APDs-derived diabetes mainly revolve around the above three aspects [11]. However, the detailed molecular mechanisms are still uncomprehensive. Glucagon-like peptide 1 receptor (GLP1R) agonist, a hotspot issue on the development of anti-hyperglycemic drugs, poses a better potential advantage than metformin in alleviating obesity and impaired glucose tolerance caused by APDs [12,13,14]. However whether GLP1/GLP1R is involved in Bre-induced MS is still unknown. Simultaneously, in order to reduce the metabolic side effects of Bre, we selected the GLP1R agonist liraglutide (LIR) for combined treatment, which could also provide reference for clinical interventions and molecular mechanism interpretation on Bre-induced metabolic disorders.
Materials and methods
Materials
Brexpiprazole, Ari, Ola, and LIR were purchased from Bide Pharmatech (Shanghai, China), Otsuka (Tokyo, Japan), Hansoh Pharma (Lianyungang, China), and Novo Nordisk (Copenhagen, Denmark), respectively. The triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), free fatty acid (FFA), and glycated serum protein (GSP) assay kits were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assays (ELISAs) kits for Glucagon-like peptide 1 (GLP1), insulin, and glucagon were from SioBestBio (Shanghai, China). A lite handheld glucometer and test strips were selected from Sinocare (Shanghai, China). All antibodies were obtained from Abclonal (Wuhan, China). The staining kits for hematoxylin eosin (H&E), periodate-Schiff (PAS), and immunohistochemistry (IHC) were from Solarbio (Beijing, China). RNA keeper tissue stabilizer, total RNA extraction reagent, reverse transcription kit for qPCR and qPCR SYBR green kit were from Vazyme (Nanjing, China). All other chemicals were purchased from Dingguo Changsheng (Beijing, China), unless otherwise specified.
Animals and treatments
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Southwest University and followed National Institutes of Health guidelines. Sprague Dawley (SD) rats, weighing 180–220 g, were purchased from Hunan SJA Laboratory Animal Co., Ltd [SCXK (Xiang) 2019-0004]. The animals were housed in an individual cage in a conditioned environment (22 ± 1 °C, 12-h light/dark cycle, free access to standard rodent chow and water) and habituated to the new environment for a week. And then they were trained to self-administer a blank sweet cookie dough pellet (0.25 g· g−1· d−1, including 30% sucrose, 32% cornstarch, 8% casein, and 30% water) for another week. All APDs administrations occurred two times a day (b.i.d.) from the beginning of the animals' light cycle to dark cycle (9:00–21:00) to maintain a stable blood concentration of the drug and minimize the sedative effects of drugs as described in our previous study [15]. Ola, Ari, or Bre was mixed in the blank sweet cookie dough pellet and given to rats by oral administration. LIR was dissolved in normal saline (NS) to a final concentration of 0.125 mg/mL and was intraperitoneally injected (i.p.) to rats once a day (q.d.).
The administration plan was established as follows.
For the first part study, to evaluate the effect of the new APD Bre on glycolipid metabolism during 28 days, rats were randomly divided into four groups, with six female and six male rats in each group: (1) the vehicle group, rats received a blank sweet cookie dough pellet; (2) the Ola group, rats were treated with Ola (1.0 mg/kg); (3) the Ari group, rats were treated with Ari (1.0 mg/kg); (4) the Bre group, rats were treated with Bre (0.5 mg/kg) (Fig. 1b).
For the second part study, to evaluate the effect of long-term Bre (42 days) exposure combined with GLP1R agonist LIR, rats were also randomly divided into four groups, with eight female rats in each group: (1) the control group, rats were i.p. injected with NS (q.d.) after receiving a blank sweet cookie dough pellet (oral, b.i.d.); (2) the Lir group, rats were co-treated with blank sweet cookie dough pellet (oral, b.i.d.) and LIR (0.125 mg/kg, i.p., q.d.); (3) the Bre group, rats were treated with Bre (0.5 mg/kg, oral, b.i.d.) and NS (i.p., q.d.); (4) the Bre + LIR group, rats were co-administered Bre (0.5 mg/kg, oral, b.i.d.) and LIR (0.125 mg/kg, i.p., q.d.) (Fig. 1c).
After completion of drug administration, all rats were fasted for 10 ± 2 h before exsanguination via the abdominal artery under pentasorbital sodium (1% dissolved in NS, i.p., 2 mL/kg) anesthesia. Then brown adipose tissue (BAT, prior to blood release), white adipose tissue (WAT, including perirenal, gonad, and mesenterium), liver, heart, spleen, kidney, pancreas, small intestine, and skeletal muscle were collected and then stored in the RNA keeper tissue stabilizer under −80 °C. While the tissue for slicing test was stored in 4% paraformaldehyde under room temperature. To calculate the viscera index, total WAT, BAT, liver, spleen, heart, and kidney were weighed when they were cut out and characterized by the ratio to body weight. Abdominal aortic blood samples were collected in coagulant-coated tubes and then centrifuged (3000 r/min, 4 °C, 10 min) to isolate the serum. The serum TG, TC, LDL, HDL, FFA, and GSP levels were measured immediately according to the kit procedures.
Measurements of body weight, blood glucose, daily food, and water consumption
The body weight, daily food, and water consumption of animals were measured every 2 days. The rats’ fasting blood glucose (FBG) was measured every week by a freestyle lite handheld glucometer after a 12-h overnight fast. The first determination was used for assignment of rats into treatment groups, and the last measurement was used for evaluation of the effect of treatment on body weight and blood glucose.
Oral glucose tolerance testing (OGTT) and insulin tolerance testing (ITT)
For glucose tolerance tests, half of rats were fasted for 12 h then treated with vehicle or drug. Two hours following treatment, blood glucose was determined (time 0) and animals were then administered intragastrically with a weight-adjusted bolus of glucose (2 g/kg; Merck, Auckland, New Zealand). For insulin tolerance tests, the other half of rats with 2 h fast were treated with vehicle or drug for 2 h and then injected s.c. with insulin (0.5 U/kg; Novo Nordisk). Blood glucose levels were determined using glucose meter at indicated time points following the glucose or insulin delivery.
Enzyme-linked immunosorbent assays (ELISAs)
The fasting and postprandial venous blood samples were collected from the tail tip at fasting (12-h overnight fasting) or postprandial (30 min after 2-g/kg glucose load) period during the OGTT test, respectively. Both, a DPP-4 inhibitor (MCE, New Jersey, USA) and a protease inhibitor cocktail (Millipore, Billerica, USA), were added to each sample before centrifugation. The concentrations of insulin, glucagon, and GLP1 in serum were measured using commercially available ELISAs kits, according to the manufacturer’s instructions.
Homeostasis model assessment (HOMA-IR)
The HOMA-IR index calculation was used to determine the insulin sensitivity during the basal, fasting state. Based on the fasting serum insulin (FSI) and FBG level, the whole body IR (HOMA-IR) can be calculated through the formula: HOMA-IR = FSI (mU/L) × FBG (mmol/L)/22.5.
H&E, PAS, and IHC staining
The liver, pancreas, or small intestine tissues were infiltrated in 4% paraformaldehyde and embedded in paraffin to prepare 3-μm slices. Deparaffinized and rehydrated slices were stained with H&E and periodic acid–schiff (PAS), respectively. For IHC analysis, tissue slices were put into the EDTA antigen retrieval solution to repair antigen and then blocked with the BSA for 30 min. The sections were incubated with GLP1 or GLP1R at 4 °C for 12 h and subsequently incubated with anti-rabbit HRP secondary antibody for 1 h. Finally, the sections were colorated by diaminobenzidine and counterstained with hematoxylin. Images of these stained sections were photographed by a microscope equipped with digital camera (Olympus, Tokyo, Japan). The liver glycogen content, islet size, and the area of protein positive staining were measured by Image J software.
Western blot analysis (WB)
According to the WB procedures mentioned previously [16], the tissues were homogenized in the RIPA lysis buffer mixed with 1% phenylmethanesulfonyl fluoride and phosphatase inhibitor cocktail (Beyotime, Shanghai, China) on the ice for 30 min, and then centrifuged (12,000 r/min, 4 °C, 10 min) to collect the supernatant. The total protein concentration of tissue extract was measured by the BCA Protein Assay Kit. Protein samples were separated on SDS-PAGE gels and then transferred to PVDF membranes (Milliproe, Billerica, USA). After the membranes were blocked by 5% skim milk for 1.5 h and incubated in relevant primary antibodies overnight at 4 °C, the corresponding HRP-conjugated anti-rabbit IgG secondary antibody was incubated with the blots for 1.5 h. The immunoreactive bands were visualized by ECL reagent (Thermo Scientific, Massachusetts, USA) using chemiluminescence developer (Clinx, Shanghai, China) and quantified via densitometric analysis using Bio-Rad Quantity One software. The data were normalized to the corresponding GAPDH levels.
Quantitative real-time PCR analysis (qPCR)
qPCR was conducted as described in our previous study [15]. Briefly, total RNA was isolated from the liver pancreas and small intestine tissue according to the protocol of the TRIzol extraction reagent. First-strand cDNA was synthesized with the Transcriptor First Strand cDNA Synthesis Kit. Each sample cDNA was performed by qPCR with the SYBR Green Select Master Mix on the MyCycler TM system (Bio-Rad, Hercules, CA, USA). The primers were synthesized by Sangon Biotech (Shanghai, China) and the sequences were listed (Table 1). Gapdh and β-actin were used as endogenous controls to normalize the relative mRNA levels, which were calculated using the 2−ΔΔCT method.
Statistical analysis
All statistical analyses were carried out with the SPSS Statistics v22.0 programme, and the results were expressed as the mean ± SEM. One-way or two-way analysis of variance was applied to analyze the data, followed by the post hoc Dunnett’s t test for multiple comparisons. P values below 0.05 (P < 0.05) were considered indicative of statistical significance.
Results
Brexpiprazole could increase weight gain and blood glucose
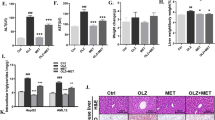
The rats treated with Ola, Ari, or Bre had different degrees of weight gain (Fig. 2a) accompanied by increased accumulation of food intake (Fig. 2b), but only Ola showed a significant difference from the 16th day to the 28th day of administration, compared to vehicle rats. The rats’ FBG was elevated obviously after 3 weeks administration of Ola or Bre, while the significant increase of FBG in Ari group only appeared at the 4th week (Fig. 2c). The viscera index of heart, spleen, kidney, and liver did not differ in each group, while the viscera index of fat in both Ola and Bre groups was higher than vehicle group (P < 0.05). Finally, all three APDs could increase the TC, FFA, and HbAlc levels (P < 0.05). Meanwhile, the content of TC could be lifted by Ola or Bre administration and HDL level was decreased by Bre with significant difference (Table 2).
a The change of accumulative body weight during 28 days. b The accumulative food intake during 28 days. c The changed fasting blood glucose during 28 days. d Oral glucose tolerance tests (OGTT) and AUC analysis for OGTT at the end (28 day) of the administration. e Insulin tolerance testing (ITT) and AUC analysis for ITT at the end (28 day) of the administration. f The basal fasting insulin resistance by means of HOMA equation. g–i The metabolic hormone levels of insulin, glucagon, and GLP1 in serum were measured by commercially available enzyme-linked immunosorbent assays. j–l The separate correlation between accumulated body weight, food intake, blood glucose, and GLP1 were analyzed by SPSS software. Values are means ± SEM (n = 12). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group.
Then we investigated whether three APDs cause alterations in glucose metabolism and insulin sensitivity through OGTT (Fig. 2d) and ITT (Fig. 2e) tests. After 28 days of APDs exposure, significant impairments in glucose tolerance were observed in Ola- or Bre-treated animals. Although results of the ITT curve at each time point did not show marked variation, the area under the curve of ITT in Ola or Bre group was bigger than that in vehicle group (P < 0.05). We further assessed the basal fasting IR by means of HOMA equation. The HOMA-IR index was markedly elevated by all three APDs, indicating fasting IR occurred (P < 0.05 vs. vehicle group) (Fig. 2f).
To determine whether the effects of APDs were due to changes in metabolic hormonal levels, we measured serum hormones that regulating glucose metabolism after glucose loading (postprandial). After APDs treatment for 28 days, compared to vehicle, Ola or Bre decreased postprandial insulin secretion, which paralleled the FBG increase in these animals. Bre or Ola can decrease the postprandial GLP1 levels while they had no evident effect on glucagon (Fig. 2g–i, respectively). In addition, we separately analyzed the correlation between accumulated body weight, food intake, blood glucose, and GLP1, and found that all three indicators had a significant positive correlation with GLP1 (Fig. 2j–l, respectively).
Brexpiprazole could decrease GLP1 expression in small intestines and pancreas
The IHC staining (Fig. 3a, d) showed that positive expressions of GLP1 were only observed in pancreatic islets and intestinal villus. Both the GLP1 expressions in pancreas and small intestine were restrained by Ola or Bre and a similar effect was observed in GLP1 gene proglucagon (Gcg) by qPCR analysis (Fig. 3c, e). The pancreas and small intestine WB analysis also revealed that the protein expression of GLP1 significantly decreased after 28 days of Ola or Bre treatment, with the inhibition of CaMKIIα, AMPK, and GLP1 transcription regulator β-Catenin (Fig. 3b, d).
a The paraffin sections of small intestine underwent immunostaining for GLP1. Scare bar = 50 µm. b The protein expression levels in small intestine tissue were assessed by Western blot analysis. c The mRNA expression levels of GLP1 (Gcg) in small intestine tissue were assessed by qRT-PCR analysis. d The paraffin sections of pancreas underwent immunostaining for GLP1. Scare bar = 50 µm. e The protein expression levels of pancreas tissue were assessed by Western blot analysis. f The mRNA expression levels of GLP1 (Gcg) in pancreas tissue were assessed by qRT-PCR analysis. Values are means ± SEM (n = 12). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group.
Brexpiprazole could induce islet dysfunction
The pancreas H&E staining exhibited clear and well-organized structure of islets in the vehicle and Ari group rats, but abnormal histological changes such as vacuolization and islet size reduction were detected in both Ola and Bre groups (Fig. 4a). The pancreas IHC staining (Fig. 4b) showed that GLP1R expressed in the entire pancreas tissue was restrained by all three APDs. The pancreas WB analysis indicated that the GLP1R protein expression was significantly decreased by Bre treatment, with the inhibition of PI3K and IRβ (P < 0.05, Fig. 4c). Moreover, the mRNA levels of insulin transcription regulator Pdx1 and Creb were increased obviously in pancreatic tissue of rats treated with Ola or Bre (Fig. 4d).
a The paraffin sections of pancreas were stained with hematoxylin and eosin. Scare bar = 50 µm. b The paraffin sections of pancreas were immunostained for GLP1R. Scare bar = 50 µm. c The protein expression levels of pancreas tissue were assessed by Western blot analysis. d The mRNA expression levels of pancreas tissue were assessed by qRT-PCR analysis. Values are means ± SEM (n = 12). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group.
Brexpiprazole could induce liver and skeletal muscle IR
From the liver H&E staining, we could clearly see the vacuolization in Bre treated rats (Fig. 5a). As for the liver PAS staining (Fig. 5b), there was not significant difference in liver glycogen aggregation between APDs groups and vehicle control group. But, in the subsequent detection of protein and gene expression, we found that the expression of IR and glycogen synthesis-related factors had changed significantly by APDs. The specific results were as follows: the protein expression of GLP1R (~51% decrease of Bre, P < 0.05), IRS1, PI3K (~42% decrease of Ola, ~68% decrease of Bre, P < 0.05), p-AKT/AKT, GLUT4, and IRβ were decreased by Ola and Bre; Ari exerted no obvious inhibitory effect on the above protein expression; Bre distinctly decreased the mRNA expression of Cpt1 and Pparγ while increased the glycogen synthesis-related factors G6pase (~122% increase) and Pepck, compared to vehicle (Fig. 5c, d).
a The paraffin sections of liver were stained with hematoxylin and eosin. Scare bar = 50 µm. b The paraffin sections of liver were stained with periodic acid–Schiff. Scare bar = 50 µm. c The protein expression levels of liver tissue were assessed by Western blot analysis. d The mRNA expression levels of liver tissue were assessed by qRT-PCR analysis. e The protein expression levels of skeletal muscle tissue were assessed by Western blot analysis. Values are means ± SEM (n = 12). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group.
As for skeletal muscle IR, we also detected the changes of insulin signal transduction pathway in skeletal muscle by WB analysis. Similarly, the protein expression levels of IRS1, PI3K, p-AKT/AKT, and GLUT4 were significantly decreased by Ola or Bre (P < 0.05 vs. Control) (Fig. 5e).
LIR could reverse the abnormal metabolic parameters induced by Brexpiprazole
Long-term (42 days) Bre treatment exerted no significant changes in body weight, accumulated food intake and water intake, but increased the blood glucose (P < 0.05 vs. control) (Fig. 6a–d). The viscera weight and serum lipid levels were also examined. Compared with the control rats, there was no change in the viscera index except for the significant increase in pWAT. The serum LDL, FFA, and GSP increased as well as HDL significantly decreased in Bre alone treated rats. Introducing LIR for co-treatment could reverse the abnormal metabolic parameters induced by Bre, such as decreases in body weight, blood glucose, total WAT, pWAT, LDL (P < 0.05 vs. Bre group), and FFA levels (Table 3). The postprandial insulin and GLP1 secretion were evidently inhibited by Bre alone. After combined treatment with Bre and LIR, the postprandial serum insulin and GLP1 content obviously increased, compared with Bre treatment alone (Fig. 6e, f). Moreover, compared with control, rats with Bre administration produced abnormal glucose tolerance in OGTT, which can be remitted by LIR (Fig. 6g, h).
a The changed body weight during the 42 days of long-term treatment. b The accumulated food intake in 42 days of long-term treatment. c The accumulated water intake in 42 days of long-term treatment. d The changed fasting blood glucose during the 42 days of long-term treatment. e The fasting and postprandial insulin concentration in serum. f The fasting and postprandial GLP1 levels in serum. g, h The oral glucose tolerance tests (OGTT) and AUC analyses for OGTT after 21 days administration and after 42 days administration. Values are means ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Bre group.
LIR could ameliorate Brexpiprazole-induced islet damage in pancreas
The pancreas H&E and IHC staining displayed the reduction of islets area and GLP1 expression caused by Bre, which was improved in the presence of LIR (Fig. 7a, b). The pancreas WB analysis revealed the downregulation of GLP1 and GLP1R protein expression by Bre, while LIR could activate the GLP1R signalling pathway and the expression of downstream protein kinase A (PKA) and PI3K to protect islet against the damage of Bre (Fig. 7c).
a The paraffin sections of pancreas were stained with hematoxylin and eosin. Scare bar = 100 µm. b The immunohistochemistry analysis of GLP1 in pancreatic tissue. Scare bar = 100 µm. c The Western blot analysis for protein expressions in pancreas tissue. Values are means ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Bre group.
LIR could ameliorate Brexpiprazole-interfered insulin cascade in liver and skeletal muscle
The WB analysis in liver showed that the protein expression of GLP1R was decreased after 42 days of Bre exposure, with the inhibition of IRS1, PI3K, p-AKT/AKT (~31% decrease of Bre, ~26% decrease of B + L, P < 0.05 vs. control), and GLUT4 (~67% decrease of Bre, ~15% decrease of B + L, P < 0.05 compared to control), but those decreased expression of proteins associated with liver insulin cascade were recovered by LIR co-administration (Fig. 8a). Identically, the WB analysis in skeletal muscle showed a significant inhibition of insulin signaling PI3K/AKT pathway in the single Bre-treated rats, which were cured in rats by combined treatment with Bre and LIR (Fig. 8b).
Discussion
The present study analyzed the effect of Bre on glucolipid metabolisms in rats, with the controls of Ola and Ari. Our results showed that Ola or Bre induced weight gain, blood glucose elevation, islet dysfunction and IR, while those metabolic adverse effects of Ari were not serious. Consistent with literatures, the incidence of metabolic disorders increased after treatment initiation, indicating that the antipsychotics may be implicated [4]. Meanwhile, it has been well proved that the risk of developing diabetes varys with different antipsychotics in plenty of studies. There is consistently higher reported diabetes risks in people taking Ola or clozapine, with the lowest risks associated with Ari [17]. An increased risk of diabetes was found in one of the four Ari studies and seven of nine Ola studies [7, 18,19,20,21]. In our study, Bre showed a more significant adverse reaction on glycolipid metabolism than Ari, but the reason was not well clear, maybe attributing to the structural modification of dichloro substituent replaced by cycloolefin on benzene ring, leading to different target affinity between Ari and Bre [22]. It may provide reference for the design synthesis and structural transformation of APDs.
The viscera indices except for fat were not affected by APDs, suggesting that the test dose of APDs can promote fat accumulation without producing toxic effects on other viscera. The content of APDs in the sweet cookie dough pellet was adjusted according to body weight, as well as the clinical administration dosage by equivalent dose conversion coefficients of human and rat based on body surface area during the course of the study to maintain the daily Ola, Ari, or Bre intakes of, respectively, 1, 1, 0.5 mg/kg. Our previous studies had demonstrated that relatively low dose of Ola was able to effectively induce hyperglycemia and weight gain in female SD rats [23]. Furthermore, the oral administration route of self-administer two times a day has been demonstrated to maintain plasma drug level and high drug compliance in the human therapeutic range [24].
We firstly investigated the effects of APDs on weight gain, since obesity is an important pathogenic factor for dyslipidemia and diabetes. APDs induce weight gain largely through increasing appetite and food intake. Indeed in this study, APDs had an obesogenic effect with appetite increase. The regulation of body weight is extremely complicated and results from the coordination of various hormonal and nervous signals from the gastrointestinal tract, adipose tissue, pancreas, and central nervous system to the hypothalamus, which in turn regulates feeding behavior and metabolism [25]. Therefore, we selected peripheral tissues to explore metabolic side effects, especially pancreatic tissues and liver tissues, which are closely related to insulin action.
In addition to obesity, the other two main characteristics of type-2 diabetes are IR and abnormal pancreatic β-cell function. Bre treatment possessed no significant change on body weight, but markedly increased the contents of FBG, GSP, TC, TG, and FFA, which are key drivers of IR. Uncontrolled lipolysis increases circulating FFA in adipocytes, which stimulates gluconeogenesis and induces liver IR through the accumulation of specific lipid intermediates, such as diacylglycerol. Although it was initially believed that APDs increased the diabetes risks by promoting weight gain, there is also evidence that APDs directly decrease insulin sensitivity and insulin secretion [26, 27]. Similarly, in this paper, the OGTT and ITT trails indicated significant impairments in glucose tolerance and IR after chronic Bre or Ola exposure, indicating that APDs may cause decreased insulin secretion after glucose load. We next detected the morphological changes of the pancreas and the disordered hormones secretion in the serum, which further confirmed that the insulin secretion and pancreatic islets function were damaged by APDs. Ketoacidosis representing severe insulin deficiency often occurs in the clinical adverse reaction reports of APDs [28], suggesting that APDs could lead to decreased insulin secretion. Chintoh used a high-glucose clamp experiment to insure that β-cells could not fully upregulate insulin secretion after Ola exposure. At the same time, it was also found that APDs could inhibit insulin secretion after glucose stimulation in islet cells [29].
Intriguingly, the GLP1 levels were decreased by Bre and showed significant negative correlations with weight gain and blood glucose, indicating that GLP1 plays a key role in the side reaction of Bre-mediated glucose and lipid metabolism. GLP1 is encoded by the proglucagon gene (Gcg) and is mainly produced by the intestinal L cells. The literature points out that 67% of GLP1 secreted from the intestine is rendered inactive when reaching the portal vein, and more than 90% became inactive when passing the liver. GLP1 signaling operates in a great part through paracrine fashion at the level of the islet that makes intuitive sense and pancreatic GLP1 is participated in physiological glucose regulation [30]. Therefore we not only detected the GLP1 level in small intestine, but also focused on pancreatic tissue, to investigate how Bre regulates the GLP1 expression. The pharmacological targets of Bre are multitudinous and complex, including D2R, D3R, 5HT2A, 5HT2B, M1R, H1R, and so on [22]. Bre is a potent antagonist of 5HT2A receptors, which blocks the downstream CaMKIIα signal transduction, thereby inhibiting AMPK and GLP1 transcription factor β-Catenin, and ultimately leading to the transcriptional inhibition of GLP1 [31]. While, the affinity of Ari on 5HT2A receptor was lower than that of Bre [22], which may explain the weaker effect of Ari, and the importance of 5HT2A on GLP1 expression as well as the lower risk of metabolic side effects. The incretin GLP1 reduces weight gain, blood glucose, and IR through a variety of pathways, such as promoting the insulin secretion in a glucose concentration-dependent manner, reducing the glucagon secretion from pancreatic α cells, protecting β cells through stimulating the cell proliferation [32], and inhibiting gastrointestinal motility to inhibit appetite [33]. It has been reported that Ola can significantly reduce the content of GLP1 and insulin after 1 h of acute Ola administration, and GLP1 receptor agonists can reverse the metabolic diseases caused by Ola [34, 35]. But there are few reports on the molecular mechanism of GLP1 in the APDs’ adverse reaction of glucolipid metabolism. In order to illustrate the molecular mechanisms of Bre-mediated metabolic disorder and the role of GLP1/GLP1R in it, we first detected the expression of GLP1R in pancreatic tissue. The results showed that three APDs significantly inhibited the GLP1R protein expression. The GLP1R is dependent on the GLP1 peptides to maintain glucose homeostasis [36], so we focused on how APDs regulate GLP1 expression. The molecular signals related to insulin synthesis and cell apoptosis were inhibited by Bre, restating the previous results of reduced insulin secretion and destroyed pancreatic islets. GLP1 secreted from α cells acts on GLP1R on neighboring cells to regulate insulin secretion through a classic G protein receptor signal activation pathway. After GLP1 binds to GLP1R on the β cell surface, the downstream cAMP-dependent PKA was activated [30]. The subsequent activation of downstream signal PI3K, on the one hand, increases the expression of PDX-1 binding to the cAMP response element site of the insulin gene promoter (CREB), thereby promoting the transcription activity of β-cell insulin gene [37], on the other hand, inhibits the expression of pro-apoptotic protein Bax, thereby inhibiting the apoptosis of islet β-cells [38].
In IR, the response of the peripheral target tissues (including liver, skeletal muscle and adipose tissue) to insulin is significantly decreased. The liver is one of the main organs for IR and insulin effector, while skeletal muscle IR is believed to be more important than the liver. Hence we simultaneously detected the changes of insulin signal transduction pathway in liver and skeletal muscle. PI3K/AKT is a classical pathway of insulin cascade to regulate metabolism. APDs inhibit the insulin signal transduction pathway of target cells, including suppressing downstream factors IRS1, PI3K activation, and AKT phosphorylation, subsequently interfering GLUT4 translocation to the cell membrane, decreasing insulin-stimulated glucose uptake, increasing gluconeogenesis through the activation of the marker gene Pepck and G6pase, and ultimately causing IR and blood glucose elevation [39, 40]. It has been reported that the inflammatory marker C-reactive protein (a nonspecific acute phase reactant) is commonly elevated in human insulin resistant states, which in turn inhibits the insulin signaling cascade and ultimately damages the islet β-cell [41].
The above results have indicated that Bre presented the same metabolic side effects as Ola through suppressing the expression of GLP1/GLP1R and its downstream signals. However, Ari had little effect in inhibiting GLP1/GLP1R signaling, which was consistent with the weakest reactivity on the metabolic derangement in Ari group. It was not difficult to draw a conclusion that the GLP1/GLP1R signal played an important role in Bre-derived glucolipid metabolism disorder.
In order to further manifest that GLP1 is indeed involved in the glucolipid side effects mediated by Bre, and to provide a combination intervention program for the treatment of Bre-induced metabolic disorders, we next conducted the second part co-administration study, to see whether the GLP1R agonist LIR could reverse the glucolipid metabolism disorder induced by Bre. The first part study found that female rats showed stronger metabolic risk than male rats (Fig. S1), which is in line with the literature report [42,43,44]. So we selected female rats as the research object for this part. The prevalence of diabetes was higher in women and those with multiple episode of psychosis, which can be explained by the estrogen action. A research about the mechanism of gender differences in the metabolic side effects of APDs is in progress in our lab. The present study demonstrated that LIR could improve the metabolic abnormalities from long-term Bre administration. In recent years, a variety of intervention drugs have been used to minimize the MS induced by Ola or other APDs, such as metformin [45], berberine [46], betahistine [47], and topiramate [48, 49]. LIR, as a profound and valuable drug for the treatment of diabetes, has been well verified by animal experiments and clinical trialson its effectiveness and safety for the treatment of MS induced by APDs [13, 50, 51].
In conclusion, Bre can induce weight gain, glucose metabolism disorder, islet dysfunction, and liver IR as similar as Ola. The molecular mechanism may be that Bre inhibited GLP1/GLP1R expressions and consequently suppressed PI3K/AKT signal activation, leading to decreased insulin secretion, liver IR, and ultimately dyslipidemia and glucose excursions (Fig. 9). Although Bre is a structural analog of Ari, it has more obvious potential risk of developing metabolism disorder than Ari. Bre should be carefully and critically prescribed, and at the same time, changes in patients’ metabolic index should be monitored dynamically during Bre administration. It is better to select Ari with equivalent efficacy of Bre for patients with a greater metabolic risk . Moreover, LIR is an effective and safe agent to ameliorate Bre-induced glycolipid metabolism disorder.
Brexpiprazole suppressed GLP1 expression by inhibiting CaMKIIα/AMPK/β-Catenin in small intestine and pancreas, leading to the decreased insulin secretion by reducing GLP1R, PKA and CREB expression in the pancreas. Moreover, the consequent restraint of IRS1, PI3K, p-Akt and GLUT4 in the liver and skeletal muscle, blocked insulin signal transduction, and eventually induced insulin resistance and metabolic syndrome.
References
Llorca PM, Lançon C, Hartry A, Brown TM, Dibenedetti DB, Kamat SA, et al. Assessing the burden of treatment-emergent adverse events associated with atypical antipsychotic medications. BMC Psychiatry. 2017;17:7.
Mamakou V, Thanopoulou A, Gonidakis F, Tentolouris N, Kontaxakis V. Schizophrenia and type 2 diabetes mellitus. Psychiatriki. 2018;29:64–3.
Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–48.
Fernandez E, Miller B, Bernardo M, Donner T, Kirkpatrick B. Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res. 2008;98:302–6.
Pillinger T, Mccutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77.
Anyanwagu U, Idris I, Donnelly R. Drug-induced diabetes mellitus: evidence for Statins and other drugs affecting glucose metabolism. Clin Pharmacol Ther. 2016;99:390–400.
Annamalai A, Kosir U, Tek C. Prevalence of obesity and diabetes in patients with schizophrenia. World J Diabetes. 2017;8:390–6.
Lieberman JA, Stroup TS, Mcevoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23.
Markovic M, Gallipani A, Patel KH, Maroney M. Brexpiprazole. Ann Pharmacother. 2017;51:315–22.
Kane JM, Skuban A, Ouyang J, Hobart M, Pfister S, Mcquade RD, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164:127–35.
Holt RIG. Association between antipsychotic medication use and diabetes. Curr Diab Rep. 2019;19:96.
Siskind DJ, Leung J, Russell AW, Wysoczanski D, Kisely S. Metformin for Clozapine associated obesity: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0156208.
Svensson CK, Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiat Scand. 2019;139:26–36.
Siskind D, Hahn M. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21:293–302.
Liu XM, Zhao XM, Deng C, Zeng YP, Hu CH. Simvastatin improves olanzapine-induced dyslipidemia in rats through inhibiting hepatic mTOR signaling pathway. Acta Pharmacol Sin. 2019;40:1049–57.
Li D, Wang X, Huang Q, Li S, Zhou Y, Li Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-κB pathway in vivo and in vitro. Redox Biol. 2018;15:62–73.
Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–74.
Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–4.
Kessing LV, Thomsen AF, Mogensen UB, Andersen PK. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197:266–71.
Hirsch L, Yang J, Bresee L, Jette N, Patten S, Pringsheim T. Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf. 2017;40:771–81.
Citrome L, Kalsekar I, Baker RA, Hebden T. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr Med Res Opin. 2014;30:1629–41.
Stahl SM. Mechanism of action of brexpiprazole: comparison with aripiprazole. CNS Spectr. 2016;21:1–6.
Liu X, Feng X, Deng C, Liu L, Zeng Y, Hu CH. Brown adipose tissue activity is modulated in olanzapine-treated young rats by simvastatin. BMC Pharmacol Toxicol. 2020;21:48.
Pan B, Lian J, Huang XF, Deng C. Aripiprazole increases the PKA signalling and expression of the GABAA receptor and CREB1 in the nucleus accumbens of rats. J Mol Neurosci. 2016;59:36–47.
Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment—pharmacological mechanisms. Pharmacol Ther. 2010;125:169–79.
Chen J, Huang XF, Shao R, Chen C, Deng C. Molecular mechanisms of antipsychotic drug-induced diabetes. Front Neurosci. 2017;11:643.
Boyda HN, Tse L, Procyshyn RM, Wong D, Wu TK, Pang CC, et al. A parametric study of the acute effects of antipsychotic drugs on glucose sensitivity in an animal model. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:945–54.
Polcwiartek C, Vang T, Bruhn CH, Hashemi N, Rosenzweig M, Nielsen J. Diabetic ketoacidosis in patients exposed to antipsychotics: a systematic literature review and analysis of Danish adverse drug event reports. Psychopharmacology. 2016;233:3663–72.
Chintoh AF, Mann SW, Lam L, Giacca A, Fletcher P, Nobrega J, et al. Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophr Res. 2009;108:127–33.
Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim KS, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 2017;25:927–34.
García-Martínez JM, Chocarro-Calvo A, Moya CM, García-Jiménez C. WNT/β-catenin increases the production of incretins by entero-endocrine cells. Diabetologia. 2009;52:1913–24.
Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–6.
Ahrén B. GLP-1 for type 2 diabetes. Exp Cell Res. 2011;317:1239–45.
Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74:719–28.
Babic I, Gorak A, Engel M, Sellers D, Else P, Osborne AL, et al. Liraglutide prevents metabolic side-effects and improves recognition and working memory during antipsychotic treatment in rats. J Psychopharmacol. 2018;32:578–90.
Meloni AR, Deyoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15:15–7.
Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002;51:691–8.
Trümper J, Ross D, Jahr H, Brendel MD, Göke R, Hörsch D. The Rap-B-Raf signalling pathway is activated by glucose and glucagon-like peptide-1 in human islet cells. Diabetologia. 2005;48:1534–40.
Garabadu D, Krishnamurthy S. Metformin attenuates hepatic insulin resistance in type-2 diabetic rats through PI3K/Akt/GLUT-4 signalling independent to bicuculline-sensitive GABAA receptor stimulation. Pharm Biol. 2017;55:722–8.
Rai U, Kosuru R, Prakash S, Singh SP, Birla H, Tiwari V, et al. Tetramethylpyrazine prevents diabetes by activating PI3K/Akt/GLUT-4 signalling in animal model of type-2 diabetes. Life Sci. 2019;236:116836.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801.
Treuer T, Pendlebury J, Lockman H, Bushe C, Karagianis J, Raskin J, et al. Weight gain risk factor assessment checklist: overview and recommendation for use. Neuro Endocrinol Lett. 2011;32:199–205.
Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. 2009;35:937–48.
Weston-Green K, Huang XF, Deng C. Sensitivity of the female rat to olanzapine-induced weight gain—far from the clinic? Schizophr Res. 2010;116:299–300.
Pinto EF, George B, Karia S, Andrade C. Metformin for antipsychotic-induced weight gain: statistical curiosities. J Psychopharmacol. 2017;31:514.
Li Y, Zhao X, Feng X, Liu X, Deng C, Hu CH. Berberine alleviates olanzapine-induced adipogenesis via the AMPKα-SREBP pathway in 3T3-L1 cells. Int J Mol Sci. 2016;17:1865.
Liu X, Lian J, Hu CH, Deng C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol Res. 2015;100:36–46.
Baptista T, Elfakih Y, Uzcátegui E, Sandia I, Tálamo E, Araujo De Baptista E, et al. Pharmacological management of atypical antipsychotic-induced weight gain. CNS Drugs. 2008;22:477–95.
Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35:1520–30.
Medak KD, Shamshoum H, Peppler WT, Wright DC. GLP1 receptor agonism protects against acute olanzapine-induced hyperglycemia. Am J Physiol Endocrinol Metab. 2020;319:E1101–11.
Babic I, Sellers D, Else PL, Nealon J, Osborne AL, Pai N, et al. Effect of liraglutide on neural and peripheral markers of metabolic function during antipsychotic treatment in rats. J Psychopharmacol. 2021;35:284–302.
Acknowledgements
This work was financially supported by grants by Chongqing Natural Science Foundation, China (cstc2020jcyj-msxmX0424), Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2018089).
Author information
Authors and Affiliations
Contributions
CHH and DJL managed the literature searches and designed the experiments; DJL, QY, and LL acquired data and drafted the article; XML and KKC interpreted data and revised the article; all authors approved the final version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Dj., Yue, Q., Liu, L. et al. Brexpiprazole caused glycolipid metabolic disorder by inhibiting GLP1/GLP1R signaling in rats. Acta Pharmacol Sin 42, 1267–1279 (2021). https://doi.org/10.1038/s41401-021-00680-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00680-x