Abstract
The kinase FLT3 internal tandem duplication (FLT3-ITD) is related to poor clinical outcomes of acute myeloid leukemia (AML). FLT3 inhibitors have provided novel strategies for the treatment of FLT3-ITD-positive AML. But they are limited by rapid development of acquired resistance and refractory in monotherapy. Recent evidence shows that inducing the degradation of FLT3-mutated protein is an attractive strategy for the treatment of FLT3-ITD-positive AML, especially those with FLT3 inhibitor resistance. In this study we identified Wu-5 as a novel USP10 inhibitor inducing the degradation of FLT3-mutated protein. We showed that Wu-5 selectively inhibited the viability of FLT3 inhibitor-sensitive (MV4-11, Molm13) and -resistant (MV4-11R) FLT3-ITD-positive AML cells with IC50 of 3.794, 5.056, and 8.386 μM, respectively. Wu-5 (1−10 μM) dose-dependently induced apoptosis of MV4-11, Molm13, and MV4-11R cells through the proteasome-mediated degradation of FLT3-ITD. We further demonstrated that Wu-5 directly interacted with and inactivated USP10, the deubiquitinase for FLT3-ITD in vitro (IC50 value = 8.3 µM) and in FLT3-ITD-positive AML cells. Overexpression of USP10 abrogated Wu-5-induced FLT3-ITD degradation and cell death. Also, the combined treatment of Wu-5 and crenolanib produced synergistic cell death in FLT3-ITD-positive cells via the reduction of both FLT3 and AMPKα proteins. In support of this, AMPKα inhibitor compound C synergistically enhanced the anti-leukemia effect of crenolanib, while AMPKα activator metformin inhibited the anti-leukemia effect of crenolanib. In summary, we demonstrate that Wu-5, a novel USP10 inhibitor, can overcome FLT3 inhibitor resistance and synergistically enhance the anti-AML effect of crenolanib through targeting FLT3 and AMPKα pathway.
Similar content being viewed by others
Introduction
Fms-related tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase expressed on hematopoietic cells. FLT3 plays a critical role in both normal and malignant hematopoiesis [1, 2]. Activating mutations of FLT3 can be found in ~30% of acute myeloid leukemia (AML) cases [3]. There are two major FLT3 activating mutations: the internal tandem duplication (ITD) in the juxtamembrane domain of the tyrosine kinase and point mutations in the tyrosine kinase domain, typically at the D835 residue. FLT3-ITD is the most common form of FLT3 mutation, with an incidence rate of 15%–35% of patients with AML [4, 5]. This mutation causes the constitutive activation of FLT3 and therefore activates multiple intracellular signaling molecules, such as PI3K/AKT and MAPK/ERK, which in turn promote the proliferation and inhibit the apoptosis of leukemia cells [6,7,8]. Several studies have demonstrated that patients with FLT3-ITD exhibit a high relapse rate, poor clinical prognosis, and lower overall survival [3, 9, 10]. FLT3-ITD is a valid target for the treatment of FLT3-ITD-positive AML. The first generation of FLT3 inhibitors, such as midostaurin and sorafenib, are relatively nonspecific for FLT3 and usually inhibit a variety of kinases, such as KIT and PDGFR. Off-target inhibition may be associated with increased toxicity and modest clinical benefit. Second-generation inhibitors, such as crenolanib and quizartinib, are more selective for FLT3 than are first-generation inhibitors [11, 12]; however, they are limited by the rapid development of acquired resistance and cancer that is refractory to monotherapy [13,14,15]. Therefore, novel strategies are required to improve the efficacy of FLT3 inhibitors.
In addition to directly inhibiting the kinase activity of FLT3, the induction of FLT3-ITD degradation is emerging as a novel strategy to target FLT3-ITD. FLT3 can be degraded by the ubiquitin-proteasome or lysosome pathway. C-Cbl is an E3 ubiquitin ligase that ubiquitinates and downregulates FLT3 [16]. However, the heterozygous deletion of c-Cbl in some FLT3-mutated cells, such as Molm13 cells [17], results in the stabilization of FLT3-ITD [18, 19]. USP10 deubiquitinates and stabilizes FLT3-ITD but not wild-type FLT3. Inhibiting USP10 causes the degradation of mutant FLT3, thus overcoming drug resistance [20]. In addition, the USP9X inhibitor WP1130 has also been reported to induce the degradation of FLT3-ITD [21]. The combination of homoharringtonine and the heat shock protein 90 inhibitor IPI504 exerted antitumor effects by reducing total FLT3 protein levels via degradation [22]. Compared with traditional FLT3 inhibitors, reducing total FLT3 protein not only inhibits the activity of FLT3 but also eradicates its kinase-independent activity. Therefore, inducing the degradation of the FLT3-mutated protein is an attractive strategy for the treatment of FLT3-ITD-positive AML, especially for those showing FLT3 inhibitor resistance [23].
In this study, we demonstrate that Wu-5 selectively induces the apoptosis and overcomes FLT3 inhibitor resistance of FLT3-ITD-positive cell lines and primary FLT3-ITD-positive AML cells. We also demonstrate that Wu-5 is a novel USP10 inhibitor. The combination of Wu-5 with crenolanib has a synergistic effect on FLT3-ITD-positive cells because both the FLT3 and AMPKα pathways are inhibited.
Materials and methods
Compounds and antibodies
Wu-5 was identified from an in-house compound library. The following antibodies were used in this study: anti-Caspase-3 (Cell Signaling Technology, 9662); anti-PARP1 (Cell Signaling Technology, 9532); anti-FLT3 (Cell Signaling Technology, 3462S); anti-P-AKT (Cell Signaling Technology, 4060S); anti-P-ERK (Cell Signaling Technology, 4370S); anti-USP10 (Santa Cruz, sc-365828); anti-AMPKα (Cell Signaling Technology, 5832S); anti-USP5 (Cell Signaling Technology, 12577S); anti-c-myc (Cell Signaling Technology, 18583); anti-β-catenin (Cell Signaling Technology, 8480S); β-Actin-HRP (conjugated) (Proteintech, HRP-60008); anti-rabbit IgG, HRP-linked (Cell Signaling Technology, 7074); and anti-mouse IgG, HRP-linked (Cell Signaling Technology, 7076).
Cell culture
The HL60 (WT-FLT3), MV4-11 (FLT3-ITD), Molm13 (FLT3-ITD), and MV4-11-R (FLT3-ITD with crenolanib resistance) cell lines were cultured in Iscove’s modified Dulbecco’s medium containing 10% FBS. U937 (WT-FLT3) and FLT3-ITD transfected Ba/F3 cells were cultured in RPMI-1640 containing 10% FBS. Parent Ba/F3 cells were cultured in 90% RPMI-1640 medium containing 10% FBS and 10 ng/mL IL-3. All the culture media contained 100 U/mL penicillin and 100 g/mL streptomycin. The cells were cultured at 37 °C in a humidified incubator with 5% CO2 and 95% air.
Transfection
USP10 plasmids were purchased from Addgene (#22543). The FLT3-ITD plasmid was kindly provided by Professor Rui-bao Ren from Ruijin Hospital, Shanghai. PLKO-shUSP10-1 and PLKO-shUSP10-2 plasmids were obtained from the DNA library of Shanghai Jiao Tong University School of Medicine. The retroviral vector PBABE-Puro and packaging vectors Gag-Pol and VSVG and lentiviral packaging plasmids PXPAX2 and PMD2G were developed in our laboratory. The USP10 genes were cloned into a PBABE-Puro vector. USP10, FLT3-ITD, or PBABE-Puro was cotransfected with Gag-Pol and VSVG into HEK293T cells. PLKO-shUSP10-1, PLKO-shUSP10-2, or PLKO-Puro was cotransfected with GPXPAX2 and PMD2G into HEK293T cells. The viral supernatants were collected 36 h after transfection, and used with 5 μg/mL polybrene to infect MV4-11 cells. After 48 h, stably transfected cells were selected with puromycin. The transfection efficiency was confirmed by Western blotting.
Establishment of the crenolanib-resistant MV4-11 cell line
MV4-11 cells were seeded onto 12-well plates (106 cells/well) and treated with crenolanib at different concentrations (3–20 nM) for 48 h. The medium containing the drug was then removed, and then, the cells were incubated in drug-free medium for another 72 h. The cells resistant to high concentrations of crenolanib were collected and plated on a new 12-well plate and treated with higher concentrations of crenolanib for another 48 h. These steps were repeated until the cells were resistant to 100 nM crenolanib.
Mononuclear cell separation
Normal human bone marrow samples were obtained from Shanghai General Hospital. FLT3-ITD-positive and FLT3-ITD-negative AML bone marrow samples were obtained from Shanghai Tongren Hospital. Bone marrow mononuclear cells were isolated as described previously [24].
Western blotting
MV4-11, Molm13, and MV4-11-R cells were treated with Wu-5 for 24 h, and then, the cells were harvested and washed with ice-cold PBS. The cells were lysed using 2× SDS (1 M Tris-HCl, pH 6.8; 0.08 mM DTT; 10% sodium deoxycholate; and 50% glycerol). Cell lysates were separated on 8%–12% SDS-PAGE gels, transferred to nitrocellulose (NC) membranes and probed with primary antibodies.
Cellular thermal shift assay (CETSA)
To evaluate the dose effect of Wu-5 on the thermal stability of USP10, cell lysates were incubated with different concentrations of Wu-5 (0–200 µM) at room temperature for 40 min, heated at 52 °C (Veriti Thermal Cycler, Applied Biosystems/Life Technologies) for 3 min, and cooled for 3 min at 25 °C. Finally, the lysates were centrifuged at 20,000 × g for 20 min at 4 °C. To determine the temperature effect on Wu-5 mediation of USP10 thermal stability, the cellular supernatants were divided into two aliquots. One aliquot was treated with DMSO, and the other aliquot was treated with Wu-5 (100 μM). After 40 min of incubation at room temperature, the respective lysates were divided into separate aliquots (30 μL) and heated individually at different temperatures for 3 min. The supernatants were transferred to a new microtube, lysed with 2× SDS at 99 °C for 10 min and analyzed by Western blotting.
Deubiquitinating enzyme (DUB) activity assay
Recombinant human His-USP10 protein was purchased from Boston Biochem (01870119A). The GST-UbA52 and USP5 proteins were purified in our laboratory, and DUB activity was measured as described previously [25].
DUB-labeling assay
HA-Ub-VS was purchased from Boston Biochem (28333619 A). MV4-11 cells were treated with Wu-5 (20 μM) for 4 h and washed twice with ice-cold PBS. The cell pellets were resuspended in 50 μL of cold cell lysis buffer and lysed by sonication. The lysate was centrifuged at 20,000 × g for 15 min at 4 °C. Then, the protein concentration in the supernatant was determined by the BCA method. Fifty micrograms of the lysate protein was incubated with 2.5 μM HA-Ub-VS in a total volume of 50 μL of DUB assay buffer at 37 °C for 1 h, boiled in reducing sample buffer, and resolved by SDS-PAGE. After the protein was transferred to an NC membrane, immunoblot analysis was performed with anti-HA (clone HA-7, Sigma-Aldrich), anti-USP10, or anti-USP5 antibodies.
Apoptosis assays
MV4-11 cells were seeded onto 12-well plates (106 cells/well) and treated with Wu-5 for 24 or 48 h or in combination with crenolanib for 48 h. Then, 5 × 105 cells were harvested and washed twice with cold PBS. The cells were washed with 1× binding buffer and stained with 10 μg/mL Annexin V-FITC for 15 min and 5 μg/mL propidium iodide (PI) for 5 min at room temperature. The cells were washed with cold PBS for flow cytometric analysis. LYSIS II software (BD Biosciences) was used to analyze the flow cytometry data.
Quantitative real-time polymerase chain reaction
MV4-11 and Molm13 cells were treated with Wu-5 for 24 h, harvested and washed twice with PBS. Cellular mRNA was extracted with an EZ-press RNA purification kit (EZBioscience) and converted to cDNA with 4 × EZscript Reverse Transcription Mix II (EZBioscience). Real-time PCR was carried out in a 96-well plate with TaqMan probes and a 7500 FAST real-time PCR system (Thermo Fisher). The ΔCT method was used to determine the expression levels of FLT3 relative to β-actin.
Cell viability assay
MV4-11, Molm13, and MV4-11-R cells were seeded onto 12-well plates (1×106 cells/well). The cells were treated with inhibitors for 24 h. DMSO was used as a vehicle control. Then, 10 μL of Cell Counting Kit-8 (CCK-8) reagent was added to each well, and the plates were incubated for 4–6 h (5% CO2, 37 °C). The absorbance was measured at 570 nm with a VICTOR3 1420 multilabel counter (PerkinElmer). Three wells were analyzed for each condition, and the wells containing only medium, CCK-8 agents or vehicle were used as controls. Inhibitory concentration 50% (IC50) values from the CCK-8 experiments were calculated with Graphpad PrismV7.04 software.
Synergy calculations
Synergy data were analyzed with online software (https://synergyfinder.fimm.fi/synergy). Combination indexes (CIs) were obtained. CI < 1 was considered to be synergistic, CI = 1 was considered to be additive, and CI > 1 was considered antagonistic.
Statistical analyses
All experimental data are expressed as the means ± SD, and statistical analyses were performed with GraphPad PrismV7.04 software based on Student’s t tests (P < 0.05). All experiments were repeated three times.
Results
Wu-5 selectively induces the death of FLT3-ITD-positive AML cells
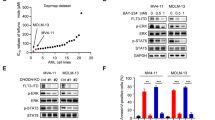
To identify compounds with anti-AML effects, we treated various cell lines with compounds from an in-house compound library. Interestingly, we found that compound Wu-5 (Fig. 1a) significantly inhibited the growth of the FLT3-ITD-positive cells, namely, MV4-11 and MV4-11-R (crenolanib-resistant) cells (Fig. S2), and Molm13 cells but had no or little effect on the proliferation of the FLT3-ITD-negative U937 and HL60 cells, as evaluated by trypan blue exclusion test (Fig. 1b). Consistent with this observation, we found that, after the introduction of the FLT3-ITD mutation, the FLT3-ITD-positive Ba/F3 cells (IL-3 independent, in the absence of IL-3) were more sensitive to Wu-5 than were the parent Ba/F3 cells (in the presence of IL-3), indicating that Wu-5 can selectively impair the function of FLT3-ITD. In addition, the presence of IL-3 partially blocked the Wu-5-induced loss of FLT3-ITD-positive Ba/F3 cell viability. Moreover, Wu-5 reduced the viability of the MV4-11, MV4-11-R, and Molm13 cells in a dose-dependent manner with IC50 values of 3.794, 8.386, and 5.056 μM, respectively (Fig. 1d). To verify the selective effect of Wu-5 on FLT3-ITD-positive AML, we treated normal human BM mononuclear cells, three primary AML cell lines with FLT3-ITD-positive expression and 1 FLT3-ITD-negative AML primary cell line with Wu-5. Wu-5 potently reduced the viability of the primary AML cells with FLT3-ITD-positive expression in a dose-dependent manner but did not affect normal human BM mononuclear cells or the FLT3-ITD-negative primary AML cells (Fig. 1e). These results indicate that Wu-5 have a selective killing effect on FLT3-ITD-positive AML cells, including those cells showing crenolanib resistance.
a Chemical structure of Wu-5. b Viability of the U937, HL60, MV4-11, Molm13, and MV4-11-R cells treated with 10 μM Wu-5 for 24, 48, and 72 h, as determined by trypan blue exclusion assay. c Viability of the Ba/F3 FLT3-ITD-positive cells (supplemented with or without 10 ng/mL IL-3) treated with different concentrations of Wu-5. d Inhibition ratios of MV4-11, Molm13, and MV4-11-R cells treated with different concentrations of Wu-5 for 24 h, as measured by CCK-8 assay. The results are presented as the means ± S.D. (n = 3). e Viability of mononuclear cells isolated from normal bone marrow (n = 2), FLT3-ITD-positive AML patient blood (n = 3), and FLT3-ITD-negative AML patient blood samples (n = 1) treated with different concentrations of Wu-5 for 24 h was determined by trypan blue exclusion assay. *P < 0.05, **P < 0.01 (Student’s t test).
Wu-5 induces apoptosis of FLT3-ITD-positive AML cells
We evaluated the effect of Wu-5 on the apoptosis of leukemia cells. MV4-11, MV4-11-R, and Molm13 cells were treated with Wu-5 at different concentrations for 24 or 48 h. The results showed that Wu-5 induced the apoptosis of MV4-11 and Molm13 in a concentration- and time-dependent manner (Fig. 2a, c, e; Fig. S3), as demonstrated by the Annexin V/PI double-staining assay. To further confirm the apoptosis-inducing effect of Wu-5 on AML cells, we used Western blotting to examine the expression of apoptosis-related markers. Wu-5 treatment decreased the levels of PARP1 and caspase3 and increased cleaved-caspase3 expression in the MV4-11 (Fig. 2b), MV4-11-R cells (Fig. 2d), and Molm13 cells (Fig. 2f). These results indicate that Wu-5 induce apoptosis of FLT3-ITD-positive AML cells.
a, c, e Flow cytometric analysis of the apoptosis ratios of the MV4-11, MV4-11-R, and Molm13 cells treated with different concentrations of Wu-5. b, d, f Western blot analysis of the expression of PARP1, caspase3, and cleaved-caspase3 in the MV4-11, MV4-11-R, and Molm13 cells treated with different concentrations of Wu-5. β-actin was used as the loading control. The results are presented as the means ± S.D. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test).
Wu-5 reduces FLT3-ITD levels
Based on the selective killing effect of Wu-5 on FLT3-ITD-positive AML cells, we assumed that it might affect the FLT3-ITD site and/or downstream signaling pathways. To prove our hypothesis, we treated MV4-11, MV4-11-R, Molm13, and HL60 cells with different concentrations of Wu-5 for 24 h and measured the levels of FLT3, P-AKT, and P-ERK by Western blot analysis. The results showed that Wu-5 decreased the level of FLT3 and simultaneously decreased the level of phosphorylation of AKT and ERK (P-AKT, P-ERK), the proteins downstream of FLT3 in the FLT3-ITD-positive cells (Fig. 3a–d). In addition, Wu-5 decreased the FLT3 protein in FLT3-ITD-positive but not in the FLT3-ITD-negative primary AML cells (Fig. 3e, f). These results indicate that Wu-5 inhibits the growth of crenolanib-sensitive and crenolanib-resistant FLT3-ITD-positive cells by decreasing the FLT3-ITD level and inactivating its downstream signaling pathways.
a–d Expression of FLT3, P-AKT, and P-ERK in the MV4-11, Molm13, MV4-11-R, and HL60 cells treated with different concentrations of Wu-5 for 24 h. e, f Expression of FLT3 and USP10 in the FLT3-ITD-positive and FLT3-ITD-negative primary AML cells treated with 10 μM Wu-5. β-actin was used as the loading control.
Wu-5 induces proteasome degradation of FLT3-ITD
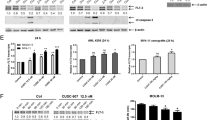
To investigate the underlying mechanism by which Wu-5 induced the downregulation of FLT3-ITD, we first examined its mRNA level by qPCR analysis. The results showed that Wu-5 did not reduce the mRNA level of FLT3-ITD (Fig. 4a). We then sought to determine whether the Wu-5-induced low expression of FLT3-ITD was caused by degradation. Interestingly, the proteasome inhibitor MG132 (5 μM) (Fig. 4b–d) but not the lysosome inhibitor CQ (20 μM) (Fig. 4e–g) partially rescued Wu-5-induced FLT3-ITD from degradation. These results suggest that Wu-5-induced FLT3-ITD degradation is mediated by the proteasome pathway.
a Relative mRNA levels of FLT3 relative to that of β-actin in the MV4-11, Molm13, and MV4-11-R cells treated with different concentrations of Wu-5 for 24 h. b–d Expression of FLT3 in the MV4-11, MV4-11-R, and Molm13 cells treated with 5 μM MG132 and 5 μM Wu-5. e–g Expression of FLT3 in the MV4-11, MV4-11-R, and Molm13 cells treated with 20 μM CQ and 5 μM Wu-5.
Wu-5 inhibits USP10 activity in vitro and interacts with USP10 in cells
The ubiquitination of FLT3-ITD is regulated by its ubiquitin E3-ligase c-Cbl and deubiquitinase USP10. However, c-Cbl is mutated in Molm13, which significantly prolongs the half-life of the FLT3-ITD in AML cells. Therefore, we hypothesized that Wu-5 might affect USP10. Using an in vitro gel-based assay, we found that Wu-5 inhibited the USP10-mediated cleavage of GST-UbA52 (Fig. 5a). The IC50 of Wu-5 on USP10 activity was 8.3 µM (Fig. 5b). However, Wu-5 (50 µM) did not affect the activity of USP5 (Fig. S4). Next, we performed CETSA to assess the binding of a drug to target proteins in cells and tissue samples [26, 27]. The results showed that, compared to the DMSO group, the incubation with Wu-5 reduced the thermal stability of USP10 in the cell lysates as the temperature increased, which was observed in both the MV4-11 cells (Fig. 5c, d) and Molm13 cells (Fig. 5e, f). Consistent with these results, Wu-5 also reduced the thermal stability of USP10 in a concentration-dependent manner (Fig. 5g–j). These results suggest that Wu-5 directly interacts with USP10 in vitro and in cells.
a Coomassie blue staining of GST-UbA52 and GST-Ub in the GST-UbA52 samples incubated with different concentrations of Wu-5 and USP10 at 37 °C for 30 min, b statistical results. c, e CETSA on USP10 in the MV4-11 and Molm13 cells treated with Wu-5 or DMSO at different temperatures, d, f statistical results. g, i CETSA on USP10 in the MV4-11 and Molm13 cells treated with different concentrations of Wu-5, h, j statistical results.
Inactivation of USP10 contributes to Wu-5-induced apoptosis
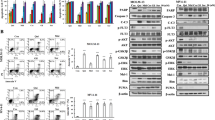
We next evaluated the effect of Wu-5 on USP10 in FLT3-ITD-positive cell lines. The results show that the Wu-5 treatment significantly downregulated the protein level of AMPKα, a downstream substrate of USP10 [28]. A slight decrease in USP10 protein was also observed (Fig. 6a–c). These results suggest that Wu-5 inhibit the activity of USP10 in cells. To evaluate the selectivity of Wu-5 on DUBs in cells, a DUB-labeling assay was performed. The results showed that Wu-5 inhibited the binding of HA-Ub-VS to USP10 but not to USP5 (Fig. 6d).
a–c Expression of USP10 and AMPKα in the MV4-11, MV4-11-R, and Molm13 cells treated with different concentrations of Wu-5 for 24 h. d HA-Ub-VS labeling assays of USP10 and USP5 in the MV4-11 cells treated with/without Wu-5. e Expression of USP10 in the MV4-11 cells transfected with PBABE-USP10. f Expression of FLT3, USP10, AMPKα, and P-ERK in the control or USP10-overexpressing MV4-11 cells treated with different concentrations of Wu-5. g Flow cytometric analysis used to the apoptosis ratios of the control group and USP10-overexpressing MV4-11 cells treated with different concentrations of Wu-5. **P < 0.01, ***P < 0.001.
To investigate the role of USP10 in Wu-5-induced death of FLT3-ITD-positive cells, USP10 was overexpressed in the MV4-11 cells (Fig. 6e). As expected, overexpression of USP10 abrogated the Wu-5-induced downregulation of the FLT3-ITD-positive protein and cell death (Fig. 6f, g and Fig. S5).
Wu-5 synergistically enhances the crenolanib-induced death of FLT3-ITD-positive AML cells via the dual inhibition of AMPKα and FLT3-ITD.
Crenolanib is a novel FLT3-ITD inhibitor used for the treatment of AML and shows significant inhibition of the growth of FLT3-ITD cells. Its IC50 values are 5.94 nM for the MV4-11 cells and 3.54 nM for the Molm13 cells (Fig. S6a, b). To test whether Wu-5 can synergize with FLT3 inhibitors to suppress the proliferation of AML cells, we treated MV4-11 and Molm13 cells with Wu-5 in the presence or absence of crenolanib. The results showed that cotreatment of Wu-5 with crenolanib synergistically inhibit the proliferation of the MV4-11 and Molm13 cells (Fig. 7a). To investigate the mechanism by which Wu-5 and crenolanib synergistically induced apoptosis, we examined the protein levels of FLT3-ITD, USP10 and their downstream substrates. Compared with the effect of the single treatment, cotreatment with Wu-5 and crenolanib reduced the protein levels of FLT3, P-AKT, P-ERK, AMPKα, β-catenin, and c-myc (Fig. 7b). With an effect similar to that of the Wu-5 treatment, knocking down USP10 reduced the protein levels of FLT3-ITD and AMPKα (Fig. 7c). These observations suggest that Wu-5 and crenolanib synergistically inhibit the USP10 and FLT3 signaling pathways. To confirm the important role of USP10 inhibition in the combination effects, USP10-overexpressing MV4-11 cells were treated with Wu-5 plus crenolanib. Compared with the control group, overexpression of USP10 abrogated Wu-5 plus crenolanib-induced loss of FLT3 and AMPKα (Fig. 7d) and apoptosis of the MV4-11 cells (Fig. 7e, Fig. S7). Next, to investigate the therapeutic potential of the combined inhibitory effect on AMPKα and FLT3-ITD, we treated MV4-11 cells by inhibiting FLT3 in the presence or absence of an AMPKα activator and inhibitor. As expected, the AMPKα activator metformin inhibited (Fig. 7f, Fig. S6c), while the AMPKα inhibitor compound C synergistically enhanced the anti-leukemic cell effect of crenolanib (Fig. 7g, Fig. S6d). These results show that targeting USP10 synergistically enhance FLT3 inhibitor-induced apoptosis.
a The combination index (CI) of Wu-5 and crenolanib in the FLT3-ITD-expressing MV4-11 and Molm13 cells (n = 2). Red indicates synergy, and green indicates antagonism. b Expression of FLT3, β-catenin, AMPKα, c-myc, P-AKT, and P-ERK in the MV4-11 cells treated with Wu-5 and/or crenolanib. c Expression of FLT3, USP10, and AMPKα in the shUSP10 MV4-11 cells. d, e Expression of FLT3, USP10, β-catenin, AMPKα, c-myc, P-AKT, and P-ERK in the control and PBABE-USP10 MV4-11 cells treated with Wu-5 and/or crenolanib, and apoptosis ratios were determined based on the flow cytometric analysis. f CI of metformin and crenolanib in MV4-11 cells. g CI of compound C and crenolanib in the MV4-11 cells. The results are presented as the means ± SD (n = 3). ***P < 0.001.
Discussion
Several new FLT3 inhibitors have been developed to treat FLT3-mutated AML. However, as a monotherapy, each FLT3 inhibitor has been limited by its incomplete and transient clinical responses and acquired resistance [29]. In this study, we demonstrated that Wu-5, a novel USP10-targeting compound, can induce the degradation of FLT3-ITD and synergistically enhance crenolanib-induced apoptosis.
FLT3 mutations, such as FLT3-ITD and FLT3-D835, stimulate constitutive activation of the FLT3-expressing tyrosine kinase [30]. Correspondingly, most FLT3 inhibitors in clinical practice aim to inhibit FLT3 activity directly. However, frequent drug resistance to FLT3 inhibitors creates difficulty for the use of this treatment [31]. Rather than inhibiting the kinase activity of oncoproteins, a novel “degradation of oncoproteins” strategy represents a new possibility for anticancer therapy as it bypasses the drug resistance problem, especially those induced by various mutations. For example, quizartinib-based PROTAC has been shown to decrease the protein level of FLT3-ITD [23]. In the case of our study, the known E3 ligase for FLT3 is c-Cbl. However, mutations in c-Cbl have been found in AML patients, which makes the activation of c-Cbl inapplicable to AML patients. Our work demonstrates that complementing this E3-ligase-based strategy, the inhibition of DUBs promotes the degradation of FLT3-ITD in both crenolanib-sensitive and crenolanib-resistant cells. Specifically, we demonstrated that Wu-5, a compound screened from our in-house compound library (Fig. S1), selectively inhibited the growth of FLT3-ITD-positive leukemia cells. Derivatives of Wu-5 have been reported to have potential therapeutic effects on human breast cancer [32, 33] and neuroprotective effects in Parkinson’s disease [34]; however, their direct targets remain unknown. Our results show that Wu-5 is a novel USP10 inhibitor, as evidenced by its inhibitory effect on purified USP10 but not USP5 in vitro and its ability to bind USP10 in cells. The selectivity of Wu-5 for USP10 was also confirmed by the DUB-labeling assay results.
Our results revealed USP10 as a novel target for the treatment of FLT3-ITD-positive leukemia cells. The role of USP10 in cancer is dependent on cell type. In liver and lung cancer, USP10 is a tumor suppressor, while in colon and prostate cancer and chronic myeloid leukemia, USP10 appears to be an oncogene [35,36,37]. However, the role of USP10 in leukemia has not yet been established. Our results support the use of USP10 as a novel target for the treatment of FLT3-ITD-positive leukemia cells from two respects: a, inhibiting USP10 by Wu-5 resulted in the degradation of FLT3-ITD, and b, overexpression of USP10 inhibited Wu-5 and crenolanib-induced cell death. Further analysis of USP10 in a large number of FLT3-mutated AML samples may address whether the expression level of USP10 has prognostic value for FLT3-mutated AML.
In our previous work, we demonstrated that the combination of arsenic trioxide and imatinib was effective in the treatment of chronic myeloid leukemia [38]. In this combination, imatinib inhibited the activity of BCR-ABL, and arsenic trioxide induced the degradation of BCR-ABL. Similar to this strategy, our present work and that of others demonstrated that the combination of an FLT3 inhibitor with an FLT3-degradation inducer was also effective [20]. Moreover, in addition to inhibiting the activation of FLT3 downstream substrates, this combination had a synergistic effect in reducing AMPKα levels. As has been reported previously that, under energy stress, USP10 removes ubiquitin from AMPKα, whereas AMPKα phosphorylates USP10 and activates USP10, forming a positive feedback loop [39]. Consistent with this finding, our results showed that Wu-5 inhibited USP10 and resulted in the reduction of AMPKα, which may further inhibited the activation of USP10. In addition, we present, for the first time, evidence that crenolanib also reduces the protein level of AMPKα. The combined effect of Wu-5 and crenolanib on AMPKα may contribute to the marked reduction in β-catenin and c-myc. Accordingly, we also demonstrated that the AMPKα inhibitor compound C and the activator metformin enhanced and inhibited the anti-leukemia activity of crenolanib, respectively. Taken together, our data lead us to propose that the highly synergistic effect of Wu-5 with crenolanib on FLT3-ITD-positive AML cells is attributed to their combined effect on FLT3-ITD and AMPKα (Fig. 8).
In summary, using Wu-5, identified as a novel USP10 inhibitor, we demonstrate that the combination of an FLT3 inhibitor and an FLT3-degradation inducer is a promising strategy to overcome FLT3 inhibitor resistance. Moreover, the combination of an AMPKα inhibitor with an FLT3 inhibitor deserves further investigation in the future.
References
Williams KM, Moore AR, Lucas PJ, Wang J, Bare CV, Gress RE. FLT3 ligand regulates thymic precursor cells and hematopoietic stem cells through interactions with CXCR4 and the marrow niche. Exp Hematol. 2017;52:40–49.
Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–39.
Lagunas-Rangel FA, Chavez-Valencia V. FLT3-ITD and its current role in acute myeloid leukaemia. Med Oncol. 2017;34:114.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Larrue C, Saland E, Boutzen H, Vergez F, David M, Joffre C, et al. Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood. 2016;127:882–92.
Bruner JK, Ma HS, Li L, Qin ACR, Rudek MA, Jones RJ, et al. Adaptation to TKI treatment reactivates ERK signaling in tyrosine kinase-driven leukemias and other malignancies. Cancer Res. 2017;77:5554–63.
Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang J, et al. ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia. 2017;31:2143–50.
Fan YC, Cao YN, Bai XS, Zhuang WF. The clinical significance of FLT3 ITD mutation on the prognosis of adult acute promyelocytic leukemia. Hematology. 2018;23:379–84.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98.
Weis TM, Marini BL, Bixby DL, Perissinotti AJ. Clinical considerations for the use of FLT3 inhibitors in acute myeloid leukemia. Crit Rev Oncol Hematol. 2019;141:125–38.
Shimada A. Hematological malignancies and molecular targeting therapy. Eur J Pharmacol. 2019;862:172641.
Skayneh H, Jishi B, Hleihel R, Hamieh M, Darwiche N, Bazarbachi A, et al. A critical review of animal models used in acute myeloid leukemia pathophysiology. Genes. 2019;10:614.
Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, et al. CAR T-cells targeting FLT3 have potent activity against FLT3−ITD+AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32:1168–79.
Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114:2984–92.
Oshikawa G, Nagao T, Wu N, Kurosu T, Miura O. c-Cbl and Cbl-b Ligases Mediate 17-Allylaminodemethoxygeldanamycin-induced degradation of autophosphorylated Flt3 kinase with internal tandem duplication through the ubiquitin proteasome pathway. J Biol Chem. 2011;286:30263–73.
Makishima H, Sugimoto Y, Szpurka H, Clemente MJ, Ng KP, Muramatsu H, et al. CBL mutation-related patterns of phosphorylation and sensitivity to tyrosine kinase inhibitors. Leukemia. 2012;26:1547–54.
Buchwald M, Pietschmann K, Muller JP, Bohmer FD, Heinzel T, Kramer OH. Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia. 2010;24:1412–21.
Taylor SJ, Thien CBF, Dagger SA, Duyvestyn JM, Grove CS, Lee BH, et al. Loss of c-Cbl E3 ubiquitin ligase activity enhances the development of myeloid leukemia in FLT3-ITD mutant mice. Exp Hematol. 2015;43:191–206.
Weisberg EL, Schauer NJ, Yang J, Lamberto I, Doherty L, Bhatt S, et al. Inhibition of USP10 induces degradation of oncogenic FLT3. Nat Chem Biol. 2017;13:1207–15.
Akiyama H, Umezawa Y, Ishida S, Okada K, Nogami A, Miura O. Inhibition of USP9X induces apoptosis in FLT3-ITD-positive AML cells cooperatively by inhibiting the mutant kinase through aggresomal translocation and inducing oxidative stress. Cancer Lett. 2019;453:84–94.
Wu ZX, Zhuang HF, Yu QF, Zhang XZ, Jiang XD, Lu XY, et al. Homoharringtonine combined with the heat shock protein 90 inhibitor IPI504 in the treatment of FLT3-ITD acute myeloid leukemia. Transl Oncol. 2019;12:801–9.
Burslem GM, Song JY, Chen X, Hines J, Crews CM. Enhancing antiproliferative activity and selectivity of a FLT-3 inhibitor by proteolysis targeting chimera conversion. J Am Chem Soc. 2018;140:16428–32.
Xu X, Huang L, Zhang Z, Tong J, Mi J, Wu Y, et al. Targeting non-oncogene ROS pathway by alantolactone in B cell acute lymphoblastic leukemia cells. Life Sci. 2019;227:153–65.
Jing B, Liu M, Yang L, Cai HY, Chen JB, Li ZX, et al. Characterization of naturally occurring pentacyclic triterpenes as novel inhibitors of deubiquitinating protease USP7 with anticancer activity in vitro. Acta Pharmacol Sin. 2018;39:492–8.
Almqvist H, Axelsson H, Jafari R. CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil. Nat Commun. 2016;7:11040. https://doi.org/10.1038/ncomms11040.
Martinez Molina D, Nordlund P. The cellular thermal shift assay: a novel biophysical assay for in situ drug target engagement and mechanistic biomarker studies. Annu Rev Pharmacol Toxicol. 2016;56:141–61.
Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, et al. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell. 2018;23:86–100.e6.
Smith CC. The growing landscape of FLT3 inhibition in AML. Hematol Am Soc Hematol Educ Program. 2019;2019:539–47.
Elyamany G, Awad M, Alsuhaibani O, Fadalla K, Al Sharif O, Al Shahrani M, et al. FLT3 internal tandem duplication and D835 mutations in patients with acute lymphoblastic leukemia and its clinical significance. Mediterr J Hematol Infect Dis. 2014;6:e2014038.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
Feng JH, Fang H, Wang XJ, Jia YP, Zhang L, Jiao J, et al. Discovery of N-hydroxy-4-(3-phenylpropanamido) benzamide derivative 5j, a novel histone deacetylase inhibitor, as a potential therapeutic agent for human breast cancer. Cancer Biol Ther. 2011;11:477–89.
Molloy ME, White BEP, Gherezghiher T, Michalsen BT, Xiong R, Patel H, et al. Novel selective estrogen mimics for the treatment of tamoxifen-resistant breast cancer. Mol Cancer Ther. 2014;13:2515–26.
Gliyazova NS, Ibeanu GC. The chemical molecule B355252 is neuroprotective in an in vitro model of Parkinson’s disease. Cell Mol Neurobiol. 2016;36:1109–22.
Liao YN, Guo ZQ, Xia XH, Liu Y, Huang CY, Jiang LL, et al. Inhibition of EGFR signaling with Spautin-1 represents a novel therapeutics for prostate cancer. J Exp Clin Cancer Res. 2019;38:157. https://doi.org/10.1186/s13046-019-1165-4.
Chen Q, Hang YY, Zhang TT, Tan L, Li SD, Jin YL. USP10 promotes proliferation and migration and inhibits apoptosis of endometrial stromal cells in endometriosis through activating the Raf-1/MEK/ERK pathwa. Am J Physiol-Cell Physiol. 2018;315:C863–C72.
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34.
Xu WB, Wei W, Yu Q, Wu C, Ye CJ, Wu YL, et al. Arsenic trioxide and bortezomib interact synergistically to induce apoptosis in chronic myelogenous leukemia cells resistant to imatinib mesylate through Bcr/Abl-dependent mechanisms. Mol Med Rep. 2014;10:1519–24.
Deng M, Yang X, Qin B, Liu T, Zhang H, Guo W, et al. Deubiquitination and activation of AMPK by USP10. Mol Cell. 2016;61:614–24.
Acknowledgements
This work was supported in part by grants from the National Key Research and Development Program of China (No. 2017YFA0505202), the National Basic Research Program of China (973 Program) (No. 2015CB910403), the National Natural Science Foundation of China (Nos. 81570118, 81700475, 81570112, 81670139, and 21602133), the National Natural Science Foundation Youths of China (No. 81700157), Innovative research team of high-level local universities in Shanghai (SSMU-ZDCX20181202).
Author information
Authors and Affiliations
Contributions
YLW and WL conceived and designed the study. MY and ZXF performed the experiments, collected data, conducted preliminary analysis of data and wrote the initial article. WWW, YZ, ZLB, ML, XHX, ZLZ, XMZ, YC, YYW, HL, HZX and YZW assisted in data analysis, manuscript writing and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, M., Fang, Zx., Wang, Ww. et al. Wu-5, a novel USP10 inhibitor, enhances crenolanib-induced FLT3-ITD-positive AML cell death via inhibiting FLT3 and AMPK pathways. Acta Pharmacol Sin 42, 604–612 (2021). https://doi.org/10.1038/s41401-020-0455-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0455-x
Keywords
This article is cited by
-
USP47 stabilizes YBX1 to promote the progression of acute myeloid leukemia
Oncogene (2024)
-
Inhibition of USP10 induces myeloma cell apoptosis by promoting cyclin D3 degradation
Acta Pharmacologica Sinica (2023)
-
The roles of ubiquitination in AML
Annals of Hematology (2023)
-
A bavachinin analog, D36, induces cell death by targeting both autophagy and apoptosis pathway in acute myeloid leukemia cells
Cancer Chemotherapy and Pharmacology (2022)
-
Deubiquitinases in hematological malignancies
Biomarker Research (2021)