Abstract
Cancer nanomedicines have shown promise in combination immunotherapy, thus far mostly preclinically but also already in clinical trials. Combining nanomedicines with immunotherapy aims to reinforce the cancer-immunity cycle, via potentiating key steps in the immune reaction cascade, namely antigen release, antigen processing, antigen presentation, and immune cell-mediated killing. Combination nano-immunotherapy can be realized via three targeting strategies, i.e., by targeting cancer cells, targeting the tumor immune microenvironment, and targeting the peripheral immune system. The clinical potential of nano-immunotherapy has recently been demonstrated in a phase III trial in which nano-albumin paclitaxel (Abraxane®) was combined with atezolizumab (Tecentriq®) for the treatment of patients suffering from advanced triple-negative breast cancer. In the present paper, besides strategies and initial (pre)clinical success stories, we also discuss several key challenges in nano-immunotherapy. Taken together, nanomedicines combined with immunotherapy are gaining significant attention, and it is anticipated that they will play an increasingly important role in clinical cancer therapy.
Similar content being viewed by others
Introduction
Nanomedicines have been extensively investigated for tumor-targeted drug delivery and reducing the toxicities/side effects of chemotherapeutic drugs [1, 2]. Tumor targeting by nanomedicines is mainly mediated by passive targeting (based on the enhanced permeability and retention effect [3, 4]) and/or active targeting [5]. The field has witnessed the success of the first commercial liposomal nanomedicine in 1995 (liposomal doxorubicin; Doxil), and afterwards, several more nanoformulations were approved by the FDA and/or EMA, including paclitaxel-loaded in albumin nanoparticles (Abraxane) which is currently one of the best-selling cancer drugs on the market [6, 7].
In recent years, the achievements of the nanomedicine field have been critically evaluated, mainly focusing on the average targeting efficiency and real clinical impact [8, 9]. This has initiated intense discussions on the current clinical utilization and future directions of nanomedicines [10,11,12,13]. Among the main future ways forward is the combination of nanomedicines with immunotherapy, a therapeutic strategy that has been extensively studied preclinically [14,15,16,17,18,19] and is also already being explored in the clinic [20]. This combination approach has broadened the applicability of nanomedicines from solely targeting tumor tissues as monotherapies to targeting multiple other organs and cell types in combination modalities [21]. In this context, a low degree of tumor accumulation for a specific nanoparticle or cancer type is not necessarily a disadvantage anymore, since nanomedicines targeting other cells and tissues may help to boost the therapeutic efficacy of combination immunotherapy, including that with checkpoint antibodies [22].
As will be outlined in this paper, the ability of nanomedicines to activate cancer immunity and improve immunotherapeutic responses holds great potential, and there are already several pieces of evidence demonstrating that nano-immunotherapy has a bright clinical future.
Opportunities of nanomedicine in immunotherapy
The interplay between cancer nanomedicine and immunotherapy has been demonstrated in multiple preclinical studies [14]. To systematically summarize such combination therapies, we have utilized the concept of “cancer-immunity cycle” [23] to showcase methods to improve immunotherapeutic outcomes [14]. Furthermore, we have proposed a simplified model which is composed of three immune targeting strategies for combination nano-immunotherapy [15].
Integration of nanomedicines in the cancer-immunity cycle
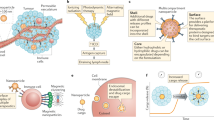
The cancer-immunity cycle is a model that describes the anticancer immune reaction cascade. As shown in Fig. 1, it is composed of four sequentially connected steps. It starts with the release of antigens from cancer cells and the antigens are then taken up and processed by antigen-presenting cells (APCs). Afterwards, they are presented to naive T cells to generate cytotoxic T cells. These cytotoxic T cells migrate via the circulation to find and kill cancer cells by releasing toxic molecules such as perforin and granzyme B. During this process of cancer cell death, more cancer antigens are released, and a new round of the immune reaction cascade can be triggered.
Integration of nanomedicines in the cancer-immunity cycle. Nanomedicines can be used in each of the steps of the cancer-immunity cycle to potentiate antigen release from cancer cells, promote antigen uptake and processing by antigen-presenting cells, and support the presentation of cancer antigens to T cells to stimulate T cells to recognize and kill cancer cells [Adapted with permission from Ref. [14]. Copyright 2019, The Royal Society of Chemistry].
Cancer nanomedicines have been utilized to initiate the release of antigens from cancer cells by loading agents that are able to induce immunogenic cell death (ICD) [24]. Examples of such agents include the classical chemotherapeutic drugs (anthracyclines, oxaliplatin, and cyclophosphamide) as well as molecules used for photodynamic/photothermal therapy [25]. A number of studies have already shown that loading such agents into nanocarriers induces more potent ICD compared with the free drug [26, 27]. An early case was demonstrated with oxaliplatin-loaded poly(lactic-co-glycolic acid) nanoparticles. In a mouse pancreatic carcinoma model, it was shown that the drug-loaded nanoparticles induced higher cell apoptosis than the free drug, and that immunoactivation effects such as enhanced effect or T cell infiltration in tumors, dendritic cell maturation, and interferon-gamma secretion were observed in mice treated with oxaliplatin-loaded nanoparticles. The therapeutic efficacy of oxaliplatin-loaded NPs was found to be significantly higher than that of the free drug [27].
In addition to inducing ICD, nanomedicines can also be used to potentiate antigen uptake, processing, and presentation. A key aspect of these three processes is the delivery of antigens and adjuvating agents to immune cells. Adjuvants, such as Toll-like receptor (TLR) agonists, target pathways in immune cells that sense pathogen/danger-associated molecular patterns, which leads to the activation of APCs [28]. Such adjuvants have shown therapeutic effects, but they are unfortunately often associated with severe side effects [29]. Therefore, nanomedicines have on several occasions been used to target adjuvants to immune cells (mainly APCs) in the lymph nodes to avoid side effects and to enhance efficacy. For example, pH-sensitive nanogels have been designed to chemically conjugate the TLR7/8 agonist imidazoquinoline. These nanogels were able to drain to lymph nodes of mice after subcutaneous injection, in which they were endocytosed by APCs and intracellularly degraded. The free polymer chains conjugated with imidazoquinoline were liberated, which triggered the TLR7/8 pathway [30]. Furthermore, nanomedicines incorporating both adjuvants and tumor antigens have also been exploited as cancer vaccines to mount an adaptive immune response against overexpressed self-antigens or neo-antigens that arise from somatic mutations in the tumor [31]. In addition to synthetic materials, cell-derived nanocarriers have shown their potential in delivering antigens for immunotherapy. For example, nanoparticles based on tumor cell membranes fused with erythrocyte membranes have been engineered, which displayed tumor antigens and resulted in efficient antigen responses in mice [32].
The final step in the cascade is cancer cell recognition and killing by cytotoxic T lymphocytes at the tumor site. This process can be hampered by multiple phenomena, including the expression of T cell inhibitory cytokines and checkpoints. To maintain the expansion and effect or functions of T cells, liposomes loaded with two cytokines (interleukins IL-15 and IL-21) were fabricated with maleimide functional groups. The liposomes were able to chemically conjugate with the surface of T cells via thiol groups on the cells and slowly release cytokines to stimulate the T cells [33]. The results showed that the liposome-treated T cells showed greater endurance in vivo in comparison to nontreated T cells, leading to improved therapeutic efficacy. Analogously, the same group also developed liposomes binding to the Thy1.1 receptor of T cells, which were also functionalized with recombinant IL-2 that binds to the IL-2 receptor on activated T cells. These dual-functional liposomes enabled the increased expansion of T cells in vivo and enhanced tumor eradication by T cells [34].
Targeting strategies in nano-immunotherapy
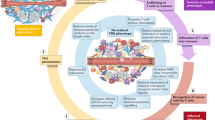
Depending on the targeting properties of nanomedicines, they can be utilized to boost cancer immunotherapy in three different ways [15] (Fig. 2). The first strategy is to target and kill cancer cells to induce specific forms of immune-activating cell death (i.e., ICD). As discussed above, such nanomedicines typically incorporate ICD-inducing chemotherapeutic drugs such as doxorubicin and oxaliplatin, and the added value of using nanomedicines in this regard is related to their ability to more efficiently deliver drugs to tumors (compared with the free drug) and at the same time to decrease accumulation and toxicity in healthy tissues.
Targeting strategies in nano-immunotherapy. Left: nanomedicines can be designed to target cancer cells and to elicit immunogenic cell death, thereby synergizing with immunotherapy. Middle: nanomedicines targeting the tumor immune microenvironment are able to beneficially modulate the immunosuppressive local environment to promote immunoactivation. Right: nanomedicines can also be developed to target the immune system outside of tumor tissues to potentiate antigen presentation in secondary lymphoid organs and to activate peripheral immune cells [Adapted with permission from Ref. [15]. Copyright 2019, American Chemical Society].
The tumor immune microenvironment (TIME) [35] plays crucial roles in cancer development, metastasis, and resistance to (immuno)therapy. The TIME contains multiple types of suppressive immune cells, such as tumor-associated macrophages (TAMs), regulatory T cells and myeloid-derived suppressor cells. In addition, there are several classes of soluble inhibitors, such as indoleamine 2,3-dioxygenase and transforming growth factor beta, which activate immunosuppressive pathways and thereby prohibit antitumor immunity. Nanomedicines have been designed to target and modulate TIME. For example, nanomedicines such as iron oxide nanoparticles [36] and CaCO3 nanoparticles [37] have been repurposed to modulate the phenotype of TAMs, leading to increased ratios of anticancer M1-like TAMs in tumor tissues. These TAM-modulating nano-immunotherapy regimens improved the efficacy of checkpoint-inhibiting antibodies in multiple cancer models in mice [37].
In addition to targeting cancer cells and modulating the TIME, nanomedicines can also target components of the peripheral immune system. Such approaches primarily target APCs in secondary lymphoid organs as well as immune cells in the circulation, including T cells. Nanomedicines have been utilized for cancer vaccination by enabling the efficient delivery of antigens and/or adjuvants to peripheral immune organs and cells. For example, therapeutic nanovaccines based on mRNAs encoding patient-specific antigens were fabricated with ionizable lipids. By tuning the physicochemical properties (size, surface charge, and stability), the nanovaccines accumulated in the spleen and transfected splenic APCs, leading to T cell responses to cancer antigens in mouse models and in patients [38]. To target T cells in the circulation, nanomedicines carrying the leukemia-specific 194–1BBz gene were designed to engineer peripheral T cells to express chimeric antigen receptor as a part of an alternative approach for CAR T therapy, which has the potential to reduce the high cost of adoptive T cell therapy [39].
Applications of nanomedicines in clinical immuno-oncology
Cancer nanomedicines have been applied in immunotherapy not only in preclinical studies but also in a number of clinical trials [20]. To date, nanomedicines, including Abraxane, Doxil, and mRNA nanovaccines, have been tested in ~150 clinical trials (Fig. 3a). Most of these trials use chemotherapeutic nanomedicines (i.e., Abraxane and Doxil). The number of clinical trials involving nanomedicines in immuno-oncology has rapidly increased in the past 5 years (Fig. 3b). The majority of current clinical trials are at stage I/II; only a few trials with Abraxane and Doxil have proceeded to phase III (Fig. 3c). Fig. 3d shows that checkpoint-inhibiting antibodies are the most commonly used immunotherapeutics in combination with nanomedicines in clinics. Importantly, Abraxane combined with atezolizumab has been approved by the FDA for the treatment of triple-negative breast cancer. The very positive outcome of this trial strongly supports the future development of combined nano-immunotherapy.
Clinical translation of nanomedicines for immunotherapy applications. a The number of clinical trials involving Abraxane, Doxil, and mRNA nanovaccines (WDVAX is a macroscale biomaterial-supported cancer vaccine and is not within the scope of the current review). b The number of immuno-oncological clinical trials involving nanomedicines conducted from 1995 to 2019. c, d Clinical stages and types of drug interventions of the respective clinical trials [Adapted with permission from Ref. [20]. Copyright 2020, John Wiley and Sons].
Challenges in the development and translation of nano-immunotherapy
There are multiple challenges that are encountered when aiming to translate immunomodulatory nanomedicines from the bench to the bedside. These are related not only to the material design but also to the clinical trial design. Several key issues in this regard are discussed below.
Nanomedicine formulations are much more complex than small molecule drug formulations, and multiple physicochemical properties, such as size, charge, structure, composition, surface properties, and colloidal stability, all play important roles in determining their in vivo performance [40]. The physicochemical properties of nanomedicines may significantly change after administration due to interactions with biological components (e.g., protein corona), which makes it difficult to predict in vivo performance [41]. Furthermore, because of their higher level of complexity, large scale production of nanomedicine formulations is more difficult than that of small molecule drugs. This not only affects nanomedicine production but also the reproducibility of preclinical studies involving nanomedicines. To address this issue, it might be useful to introduce standardized protocols for nanomedicine production, characterization, and results reporting [42]. Moreover, the scaling up of nanomedicine production can be difficult, especially for nanomedicines based on multiple different components. It is a significant challenge for complex nanomedicines to be produced at large scales for late-stage preclinical and clinical studies. Therefore, to promote the clinical translation of immunomodulatory nanomedicines, nanomedicines should be designed based on components that are as simple as possible and on production protocols that are as scalable as possible [43]. In addition, toxicities are also a critical issue that must be considered for the clinical translation of nanomedicines [44, 45].
Beyond issues related to the materials design and production, translation challenges in nanomedicine and nano-immunotherapy also entail clinical trial design. Key issues in this regard are patient recruitment and patient stratification. Patient recruitment is problematic because the numbers of chemo-immunotherapy and nano-chemo-immunotherapy trials have increased exponentially in the last 2–3 years [46]. Patient stratification is arguably one of the most important future challenges for cancer nanomedicine in general. Nanomedicines are mostly applied nonselectively to patients in whom standard treatments fail. However, it is highly questionable whether this is the right strategy to explore their clinical therapeutic potential. As has been seen for molecularly targeted therapeutics and antibodies, patients have to be screened for certain biomarkers to be included in clinical trials. Likewise, nanomedicines should only be used in the right patients, e.g., in those who show efficient accumulation of nanomedicines in tumors and/or metastases, to increase the chances of these patients showing good therapeutic responses [11].
Combination nano-immunotherapy, like other translational anticancer drug development and drug therapy fields, faces additional challenges because of gaps between preclinical and clinical studies. Firstly, as already alluded to above, large numbers of patients are already being enrolled by a rapidly increasing number of immuno-oncology trials [46] that mostly involve clinically established drugs together with novel immunotherapeutics. Therefore, it is becoming increasingly challenging to recruit sufficiently high numbers of patients in a sufficiently short period of time for inclusion in nano-immunotherapy trials. Secondly, results from clinical trials on nano-immunotherapy are not well in line with the outcomes of preclinical reports. For example, in preclinical studies, ICD-inducing nanomedicines almost always strongly potentiate checkpoint inhibition therapy. Clinically, however, various large trials involving nanomedicines have failed. The phase III JAVELIN Ovarian 200 trial, for instance, compared avelumab (Bavencio®) plus Doxil treatment to avelumab monotherapy in platinum-refractory ovarian cancer patients and failed to demonstrate a significant improvement of the therapeutic outcome by the combination treatment. One explanation for this could be that many patients who were included were not appropriate for combination treatment, as a retrospective analysis of the data showed a significant difference in response to the combination treatment between patients with positive or negative programmed death-ligand 1 expression in their tumors [20]. This exemplifies the crucial role of clinical trial design; especially in the case of nano-immunotherapy, it is critical to identify biomarkers for patient stratification and treatment outcome prediction.
Conclusions
Combining nanomedicines with immunotherapy will significantly expand their applicability in cancer therapy. Nano-immunotherapy has demonstrated greatly improved therapeutic efficacy in preclinical study setups, and positive results have also already been reported for several clinical trials which have recently been completed or are currently ongoing. To further exploit the benefits of combining nanomedicines with immunotherapeutics, several challenges need to be overcome, including issues related to (the complexity of) nanomaterial design and clinical trial design. By carefully addressing these issues, nano-immunotherapy will become more broadly applied and appreciated, contributing to better treatment options for patients in need.
References
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2007;17:20–37.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305.
Mi P, Cabral H, Kataoka K. Ligand-installed nanocarriers toward precision therapy. Adv Mater. 2019;32:1902604.
Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20–30.
He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc Chem Res. 2019;52:2445–61.
Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–21.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014.
Crommelin DJ, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–63.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–17.
Lammers T, Kiessling F, Ashford M, Hennink WE, Crommelin D, Storm G. Cancer nanomedicine: is targeting our target? Nat Rev Mater. 2016;1:16069.
McNeil SE. Evaluation of nanomedicines: stick to the basics. Nat Rev Mater. 2016;1:16073.
Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, et al. Nanomedicine and macroscale materials in immuno-oncology. Chem Soc Rev. 2019;48:351–81.
Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52:1543–54.
Wang C, Wen D, Gu Z. Cellular bioparticulates with therapeutics for cancer immunotherapy. Bioconj Chem. 2017;29:702–8.
Sang W, Zhang Z, Dai Y, Chen X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem Soc Rev. 2019;48:3771–810.
Saeed M, Gao J, Shi Y, Lammers T, Yu H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics. 2019;9:7981–8000.
Feng X, Xu W, Li Z, Song W, Ding J, Chen X. Disease immunotherapy: immunomodulatory nanosystems. Adv Sci. 2019;6:1970100.
Shi Y. Clinical translation of nanomedicine and biomaterials for cancer immunotherapy: progress and perspectives. Adv Therap. 2020. https://doi.org/10.1002/adtp.201900215.
Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–66.
Duan X, Chan C, Han W, Guo N, Weichselbaum RR, Lin W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10:1899.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111.
Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, et al. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015;17:661–70.
Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–97.
Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63.
Wu TYH, Singh M, Miller AT, De Gregorio E, Doro F, D’Oro U, et al. Rational design of small molecules as vaccine adjuvants. Sci Transl Med. 2014;6:263ra160.
Nuhn L, Vanparijs N, De Beuckelaer A, Lybaert L, Verstraete G, Deswarte K, et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc Natl Acad Sci USA. 2016;113:8098–103.
Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, et al. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;38:320–32.
Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G, et al. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5:eaaw6870.
Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41.
Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In vivo targeting of adoptively transferred T-cells with antibody-and cytokine-conjugated liposomes. J Control Release. 2013;172:426–35.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–94.
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89–97.
Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401.
Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–20.
Lane LA, Qian X, Smith AM, Nie S. Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Annu Rev Phys Chem. 2015;66:521–47.
Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC. Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev. 2012;64:1363–84.
Fari M, Björnmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, et al. Minimumin formation reporting in bio–nano experimental literature. Nat Nanotechnol. 2018;13:777–85.
Barz M. Complexity and simplification in the development of nanomedicines. Nanomedicine. 2015;10:3093–7.
Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine. 2008;4:167–71.
Wang Y, Santos A, Evdokiou A, Losic D. An overview of nanotoxicity and nanomedicine research: principles, progress and implications for cancer therapy. J Mater Chem B. 2015;3:7153–72.
Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17:854–5.
Acknowledgements
This work was supported by the European Research Council (ERC: CoG-Meta-Targeting (864121), PoC-Picelles (813086), and CoG-ImmunoBioSynth (817938)), by the China Scholarship Council, by the European Union (European Fund for Regional Development: TAKTIRA (EFRE-0801767)), by the German Research Foundation (DFG: GRK/RTG 2375 Tumor-targeted Drug Delivery (project number: 331065168) and SFB 1066), by the Aachen Interdisciplinary Center for Clinical Research (IZKF; Project O3–2), and by the Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine-Westphalia (MKW) under the Excellence Strategy of the Federal Government and the Länder (OPSF580). The work of RvdM is supported by the Netherlands Research Council (NOW: ZonMW Vici grant #016.176.622 to W.J.M. Mulder). HJY acknowledges financial support from the National Natural Science Foundation of China (51873228).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sun, Q., Bai, X., Sofias, A.M. et al. Cancer nanomedicine meets immunotherapy: opportunities and challenges. Acta Pharmacol Sin 41, 954–958 (2020). https://doi.org/10.1038/s41401-020-0448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0448-9
Keywords
This article is cited by
-
Nanoparticles mediated tumor microenvironment modulation: current advances and applications
Journal of Nanobiotechnology (2022)
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy
Acta Pharmacologica Sinica (2022)
-
Recent Advancements in Nanomedicine for ‘Cold’ Tumor Immunotherapy
Nano-Micro Letters (2021)
-
Nanomedicine and cancer immunotherapy
Acta Pharmacologica Sinica (2020)