Abstract
The programmed cell death protein 1 (PD-1) is an immune-checkpoint that negatively regulates the immune system and a key mechanism that tumors utilize to escape from immune surveillance. PD-1 antibodies can block the interaction of PD-1 with its ligands (PD-L1 and PD-L2), restore T cells activation, and elicit antitumor activity. In this paper, we reported a novel PD-1 monoclonal antibody (mAb) CS1003, which is a humanized IgG4 PD-1 mAb generated by conventional hybridoma technology, and currently being developed in multiple clinical trials as monotherapy or in combination with other anticancer agents. We showed that CS1003 bound to recombinant human, cynomolgus monkey, and mouse PD-1 with EC50 values of 0.1757, 0.2459, and 0.3664 nM, respectively. CS1003 blocked PD-1 interaction with its ligands, dose-dependently enhanced T cell proliferation and secretion of cytokines (IL-2 and IFN-γ) to the levels comparable to the reference antibody pembrolizumab. Intraperitoneal administration of CS1003 (0.1, 0.5, 2.5 mg/kg, once every 3 days) dose-dependently suppressed the growth of MC38-hPD-L1 colon cancer in hPD-1 knock-in mice. Pharmacokinetics (PK) study revealed a linear PK profile within the dose range of 2–18 mg/kg following single intravenous administration in cynomolgus monkey. These data provide a comprehensive preclinical characterization of CS1003 that supports its clinical development for cancer immunotherapy.
Similar content being viewed by others
Introduction
The tumor immune microenvironment encompasses a wide range of complex interactions between tumor cells, immune cells, including DCs, T cells, NK cells, and B cells, and the tumor stroma [1]. Effective antitumor immune responses are the result of competition between inhibitory and stimulatory signals that regulate balanced immune responses in normal tissue. Immune checkpoints are important immune regulators in maintaining immune homeostasis and preventing autoimmunity. PD-1, a protein expressed on the surface of cells, plays an important role in downregulating immune responses and promoting self-tolerance by suppressing T cell inflammatory activity [2, 3]. PD-1 was discovered and named by Tasuku Honjo and colleagues at Kyoto University in 1992 [4, 5]. To date, six anti-PD-1 antibodies have been approved for more than ten cancer indications by the FDA or NMPA. Among these, nivolumab was the first approved therapeutic anti-PD-1 monoclonal antibody (mAb) showing significant clinical efficacy in unresectable or metastatic melanoma with limited toxicity [6]. Pembrolizumab also showed significant antitumor efficacy and a good safety profile [7]. Although PD-1 mAbs demonstrate tremendous clinical success rates across multiple cancer types, 30%–60% of patients show no response to PD-1 blockade [8]. Currently, combination therapy is a key strategy to overcome the limitations of PD-1 monotherapy [9]. To evaluate the combined effect of anti-PD-1 mAbs in syngeneic mouse models with other agents, a surrogate antibody that recognizes mouse PD-1 such as RMP1–14 is often used in place of the actual therapeutic antibody. To our knowledge, none of the approved PD-1-targeting drugs recognizes mouse PD-1, and surrogate antibodies have been used for the evaluation of combination effects in syngeneic models. However, the efficacy of surrogate antibodies may not represent the actual effect in humans due to different binding epitopes.
In this paper, we report a novel human and mouse cross-reactive anti-PD-1 antibody, CS1003. It is a humanized IgG4 anti-PD-1 mAb generated by conventional hybridoma technology and developed for cancer immunotherapy. It shows comparable binding affinity, in vitro immune modulation and in vivo potency with reference antibodies. Our results suggest that CS1003 is not only an ideal therapeutic anti-PD-1 antibody candidate for cancer treatment but also a valuable tool for quickly testing combination therapy agents in syngeneic tumor models. A phase 1 study [NCT03475251] evaluating the safety, tolerability and pharmacokinetics (PK) properties of CS1003 in patients with advanced solid tumors is ongoing.
Materials and methods
Reagents
The anti-human PD-1 reference antibody pembrolizumab was purchased from Merck (Rahway, NJ, USA). CS1003, a humanized, hinge stabilized IgG4 mAb, nivolumab, and a human IgG4 isotype control antibody were synthesized by Wuxi Biologics (Shanghai, Shanghai, China). Antigens from human PD-1, PD-L1, CTLA-4, CD28 and ICOS and mouse PD-1 and PD-L1 were prepared by Wuxi Biologics (Shanghai, China). A human IFN-γ capture antibody and human IFN-γ detection antibody were purchased from Pierce Biotechnology (Rockford, IL, USA).
SPR assay
The CM5 sensor chip was activated with EDC and NHS immediately before use. An antihuman Fc IgG antibody in 10 mM NaAc (pH 4.5) was subsequently injected into Fc1-Fc4 for 420 s at a flow rate of 10 μL/min. The chip was deactivated by 1 M ethanolamine HCl. Antibodies were diluted with running buffer (1× HBS-EP + ) to 5 μg/mL and then captured onto a chip. Seven concentrations (50, 25, 12.5, 6.25, 3.125, 1.5625, and 0.78125 nM) of human PD-1 (Sino Biological) and running buffer were injected into Fc1-Fc4. Glycine (10 mM, pH 1.5) used as regeneration buffer was injected following each dissociation phase.
ELISA assay
For the binding assay, plates were precoated with fusion protein at 4 °C overnight. After 1 h of blocking, testing antibodies were added to plates. The plates were incubated at room temperature for 1 h. The binding of antibodies to immobilized proteins was detected by an HRP-labeled goat-anti-human IgG antibody. The signal was activated by dispensing the TMB substrate and then stopped by HCl. The absorbance was measured at 450 nm and 540 nm using a microplate spectrophotometer.
FACS assay
Human PD-1-expressing CHO-S cells were incubated with serial dilutions of test anti-PD-1 antibodies at 4 °C for 1 h. Testing antibodies were serially diluted (1:2) in wash buffer (1× PBS/1% BSA) starting from 16.67 nM. A FITC-labeled goat antihuman IgG was used to detect the binding of anti-PD-1 antibodies to the cells. The mean fluorescence intensity of cells was measured by a flow cytometer and analyzed by FlowJo.
Mixed lymphocyte reaction (MLR) assay
Human peripheral blood mononuclear cells (PBMCs) were freshly isolated from healthy donors using Ficoll-Paque PLUS gradient centrifugation. Isolated PBMCs were cultured in complete RPMI-1640 medium (containing 10% FBS and 1% PS) supplemented with 100 U recombinant human IL-2. CD4+ T cells were isolated from human PBMCs. Purified CD4+ T cells were cocultured with immature or mature allogeneic DCs (iDCs or mDCs). The MLR assay was set up in 96-well round-bottom plates using complete RPMI-1640 medium. CD4+ T cells, various concentrations of antibodies (166.75, 66.7, 6.67, 0.667, 0.0667, and 0.00667 nM), and allogeneic DCs were added to the plates at appropriate ratios. The plates were incubated at 37 °C in 5% CO2. IL-2 and IFN-γ levels were determined on day 3 and day 5, respectively. The cells were harvested on day 5, and CD4+ T cell proliferation was measured by 3H-TDR.
Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) assays
For the ADCC assay, activated human CD4+ T cells and serial dilutions of testing antibodies (from 66.7 nM to 0.00667 pM) were preincubated in 96-well plates for 30 min. PBMCs were then added at an effector/target ratio of 50:1. The plates were incubated at 37 °C in a 5% CO2 incubator for 4–6 h. Target cell lysis was determined by an LDH-based cytotoxicity detection kit. The Herceptin-induced ADCC effect on SK-BR-3 cells was used as a positive control.
For the CDC assay, activated human CD4+ T cells and serial dilutions of testing antibodies (from 200 nM to 0.781 nM) were mixed in 96-well plates. Human complement was added at a dilution ratio of 1:50. The plates were incubated at 37 °C in a 5% CO2 incubator for 2–3 h. Target cell lysis was determined by CellTiter-Glo. Rituxan®-induced Ramos cell lysis was used as a positive control.
In vivo efficacy study in tumor models
All animals were maintained under specific pathogen-free conditions in the animal facilities of WuXi AppTec Co., Ltd. All animal-related experiments were approved by the Animal Use and Care Committee of WuXi AppTec Co., Ltd. MC38-hPD-L1 cells were subcutaneously (s.c.) implanted into the right flank of hPD-1 knock-in female mice (Nanjing Galaxy Biopharmaceutical Co., Ltd.). Mice were randomized with mean tumor volumes calculated at ~74 mm3. Unmodified MC38 cells were s.c. implanted into the right flank of female C57BL/6 J mice (Shanghai SLAC Co., Ltd.). Mice were randomized with mean tumor volumes of ~60 mm3. Mice were dosed intraperitoneally with isotype control or test antibody once every 3 days. Tumor growth inhibition (TGI) was calculated for each group using the following formula: TGI (%) = [1-(Ti-T0)/(Vi-V0)] ×100 (Ti and Vi: the average tumor volume of the treatment group and the isotype control group, respectively, on a given day; T0 and V0: the average tumor volume of the treatment group and the isotype control group, respectively, on day 0).
PK study in cynomolgus monkeys
A single-dose PK study of CS1003 was conducted in cynomolgus monkeys at doses of 2, 6, and 18 mg/kg. Nine female and nine male cynomolgus monkeys were assigned into three treatment groups and received one intravenous infusion of CS1003 on day 0. The repeat-dose group containing six animals received four doses of CS1003 at 6 mg/kg over 4 weeks (once per week). Blood samples were collected from each animal at predose (0), 0.25 h, 0.5 h, 1 h, 4 h, 8 h, day 2 (24 h), day 4 (72 h), day 7 (144 h), day 14 (312 h), day 21 (480 h), day 28 (648 h), and day 35 (816 h) after the last dose and were processed for serum isolation. Serum concentrations of CS1003 were determined by the validated ELISA method with a dynamic range from 100 to 8000 ng/mL. Anti-drug antibody (ADA) levels were also measured with bridging ELISA.
PD-1 receptor occupancy
For PD-1 receptor occupancy, CS1003 binding to PD-1 molecules on PBMCs was assessed by flow cytometry on circulating CD3+CD8+ T cells and CD3+CD4+ T cells. Blood samples from the above monkey PK study were collected at predose (day −1), 2 h, 8 h, day 2 (24 h), day 3 (48 h), day 4 (72 h), day 7 (144 h), day 14 (312 h), and day 28 (648 h) after the single dose. Blood samples were preincubated (60 min at 4 °C) with a saturating concentration of either unlabeled hIgG4 (isotype control) or CS1003, washed extensively, and then costained with PE-conjugated antihuman IgG4, FITC-conjugated antihuman CD45, APC-conjugated antihuman CD3, PerCP-Cy5-conjugated antihuman CD4, and APC-Cy7-conjugated antihuman CD8 antibodies for 30 min at 4 °C in the dark. The erythrocytes were lysed in 2 mL 1× BD FACS Lysing Solution for 15 min at room temperature. PBMCs were washed twice and analyzed by a Becton–Dickinson FACS Canto II flow cytometer. The receptor occupancy rate (%RO) was calculated as follows: %RO = (CS1003-PE positive percent without CS1003 staining tube)/(CS1003-PE positive percent with CS1003 staining tube) ×100%.
Statistical analysis
In vitro pharmacodynamic experiments, including T cell proliferation response, IFN-γ and IL-2 secretion results were analyzed by Wilcoxon matched pairs t test with Graphpad Prism5. In vivo efficacy results were analyzed by independent sample t test using SPSS 17.0. Differences with P < 0.05 were considered significant.
Results
CS1003 selectively bound to PD-1 and blocked PD-1 interaction with its ligands
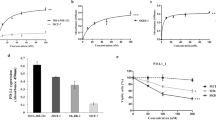
A panel of rat anti-PD-1 mAbs was generated through hybridoma technology. mAbs with favorable characteristics were further humanized. CS1003, a humanized, hinge stabilized IgG4 mAb, was selected as the lead candidate for further characterization. CS1003 potently bound to human, monkey and mouse PD-1 with KD values of 6.13, 8.74, and 3.99 nM, respectively (Table 1). The binding of CS1003 to PD-1 proteins in three species was evaluated by ELISA and flow cytometry. As shown in Fig. 1a and Supplementary Fig. S1a, CS1003 bound to human, mouse and cynomolgus monkey recombinant PD-1 with EC50 values of 0.1757, 0.3664, and 0.2459 nM, respectively. CS1003 bound to cells expressing human, cynomolgus monkey and mouse PD-1 with EC50 values of 2.20, 1.47, and 12.9 nM, respectively (Fig. 1b, Supplementary Fig. S1b, c). CS1003 did not bind to parental CHO-S cells (Supplementary Table S1). In addition, CS1003 was tested for its capability of interrupting PD-1/PD-L1 and PD-1/PD-L2 interactions. CS1003 competently inhibited the binding of human PD-1–PD-L1 and PD-L2 (Fig. 1c, Supplementary Fig. S1d), mouse PD-1–PD-L1 (Supplementary Fig. S1e) and cynomolgus monkey PD-1–PD-L1 (Supplementary Fig. S1f). The binding potency of CS1003 to human and monkey PD-1 was comparable to that of pembrolizumab; moreover, CS1003 cross-reacts with mouse PD-1. Similar to pembrolizumab, CS1003 exhibited the ability to bind human PD-1 but not other immunoglobulin superfamily proteins (CD28, CTLA-4, ICOS, and BTLA) (Fig. 1d). These results indicate that CS1003 potently binds to human, cynomolgus monkey and mouse PD-1 and efficiently blocks the PD-1/PD-L1 and PD-1/PD-L2 interactions.
a Binding of CS1003 or pembrolizumab to plate-coated recombinant human and mouse PD-1 proteins was assessed by ELISA. b Binding of CS1003 or pembrolizumab to cell surface human PD-1 was determined by FACS. c The functionality of CS1003 or pembrolizumab in blocking human PD-1/PD-L1 interactions was assessed by flow cytometry. d Binding of CS1003 or pembrolizumab to human PD-1, CD28, CTLA-4, ICOS, or BTLA was assessed by ELISA.
CS1003 enhanced T cell proliferation and cytokine secretion
Blocking the PD-1 interaction with its ligand results in enhanced T cell proliferation and cytokine secretion [10]. Therefore, CS1003 activity in regulating T cell function was measured using the MLR assay. CS1003 significantly increased IL-2 and IFN-γ levels in T cells in a dose-dependent manner similar to that of pembrolizumab (Fig. 2a, b). Using the [3H]-TDR system, CS1003 was shown to induce dose-dependent T cell proliferation with comparable efficacy to pembrolizumab (Fig. 2c). To assess whether CS1003 is capable of inducing nonspecific cytokine release in a TCR activation-dependent manner, the ability of CS1003 to induce T cell quiescence was determined. Freshly isolated human PBMCs (five donors) were incubated with 66.7 or 667 nM CS1003. The results showed that CS1003 had no significant influence on nonspecific cytokine release (data not shown). In summary, CS1003 promotes T cell proliferation and cytokine secretion in vitro.
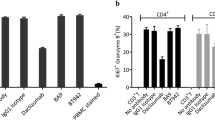
a–c The functionality of CS1003 and pembrolizumab in enhancing T cell responses was assessed using MLR, and T cell proliferation ([3H]-TDR incorporation) and effector function (IFN-γ production) were quantified. d–e CS1003 reversed Treg cell suppressive function in the MLR assay. **P < 0.01, ***P < 0.001.
CS1003 inhibited Treg cell suppressive function
PD-1-expressing Treg cells modulate T cell function in the tumor microenvironment [11]. Hence, the role of CS1003 in restoring T cell function suppressed by Treg cells was explored. CD4+CD25- effector T (Teff) cell proliferation and cytokine secretion were suppressed by allogeneic Treg cells. As shown in Fig. 2d, e, CS1003 completely restored CD4+CD25- T cell cytokine production and proliferation. Taken together, the results show that CS1003 abrogates Treg cell suppression of T cell proliferation and cytokine production.
CS1003 showed no ADCC or CDC activity
The IgG4 isotype is known to have a low effector function, which makes it ideal as a therapeutic blocking antibody [12]. CS1003 is a humanized IgG4 mAb. ADCC activities induced by CS1003 were assessed using human PBMCs as effector cells and activated human CD4+ T cells as target cells. As demonstrated in Fig. 3a, CS1003 did not induce ADCC activities on activated human CD4+ T cells, avoiding potential damage to PD-1-positive lymphocytes. The CDC activities induced by CS1003 on activated human CD4+ T cells were further evaluated. As shown in Fig. 3b, compared with the positive control rituximab, CS1003 had no apparent CDC activity within the concentration range of 0.781–200 nM. The ADCC and CDC activities of CS1003 were comparable to those of pembrolizumab (Supplementary Fig. S2). In summary, no ADCC or CDC activity was observed using CS1003.
CS1003 significantly inhibited MC38-hPD-L1 and MC38 tumor growth
The in vivo antitumor efficacy of CS1003 was evaluated using the MC38-hPD-L1 colon cancer model in hPD-1 knock-in mice. Mean tumor volumes were calculated on day 21 post-treatment (Fig. 4a), and the tumor images are shown in Fig. 4b. Compared with the isotype control, CS1003 exhibited a significant tumor growth inhibition (TGI) of 82.0%, 71.1% and 101.7% at 0.1, 0.5, and 2.5 mg/kg, respectively. In this study, nivolumab showed a TGI of 89.3% at 2.5 mg/kg, suggesting that the antitumor effect of CS1003 was comparable to that of nivolumab. No abnormal body weight changes or signs of toxicity were observed throughout the study. CS1003 also demonstrated a significant TGI effect with a TGI of 98.2% at 3 mg/kg in the unmodified MC38 model (Fig. 4c). Hence, CS1003 showed strong in vivo efficacy in both the parental MC38 model and the MC38-hPD-L1 tumor model.
CS1003 showed typical PK profiling and high receptor occupancy
A pharmacokinetics study of CS1003 was conducted in cynomolgus monkeys following a single intravenous infusion at doses of 2, 6, and 18 mg/kg. As shown in Fig. 5a and Table 2, the average initial concentration (C0) values were 58.9, 178, and 492 μg/mL and the AUC0-last values were 331, 1070, and 4580 μg•day/mL for animals in the 2, 6, and 18 mg/kg dose groups, respectively. Thus, the systemic exposure of CS1003 increased dose proportionally within the dose range from 2 to 18 mg/kg. The average serum clearance (CL) values were 6.80, 6.55, and 3.98 mL·d−1·kg−1 and the elimination half-life (T1/2) values were 6.36, 5.67, and 11.5 days in the 2, 6, and 18 mg/kg dose groups, respectively. The volume of distribution at steady-state (Vdss) was similar across groups, in the range from 0.0514 to 0.0631 L/kg. No obvious sex difference was observed in systemic exposure and the calculated PK parameters at the tested doses.
In the repeated dose PK study, following the 4th iv administration of CS1003 at 6 mg/kg, the average C0 and AUClast values were comparable with those of the single administration, suggesting no marked accumulation after the 4th dosing in either sex.
ADAs were detected from 312 h postdose in the 2 and 6 mg/kg single-dose groups and from 648 h postdose in the 18 mg/kg group. The ADA positive rate was 36.7% at 2 mg/kg, 73.3% at 6 mg/kg and 10.0% at 18 mg/kg. In the repeated dose group, the positive rate was 30%. The decrease in the ADA positive rate in the high-dose group might be due to drug tolerance when the drug concentration is high. In the repeated dose group, the ADA positive rate was 30% after the 4th dose of CS1003. Overall, the immunogenicity of CS1003 in cynomolgus monkeys was lower than that reported in previous data for pembrolizumab and nivolumab.
Receptor occupancy is an important index for anti-PD-1 mAb efficacy evaluation [13]. As shown in Fig. 5b, c, the receptor occupancy rate quickly increased following single iv administration of CS1003 at 2, 6, and 18 mg/kg and was saturated at 24 h postdose in all groups until day 14 (312 h). Decreased receptor occupancy was noted in most animals in the 2 mg/kg and 6 mg/kg dose groups on day 28, but the averaged receptor occupancy rate remained above 50%. However, in the 18 mg/kg dose group, receptor occupancy was still saturated on day 28.
Discussion
The application of immune checkpoint inhibitors has changed the paradigm in cancer treatment. Anti-PD-1 antibodies, including nivolumab, pembrolizumab, cemiplimab, JS-001, sintilimab, and camrelizumab, have been approved or are being evaluated for the treatment of more than ten cancer types, such as metastatic melanoma, non-small-cell lung cancer, head and neck cancer, Hodgkin’s lymphoma, urothelial carcinoma, gastric cancer, cervical cancer, hepatocellular carcinoma, Merkel cell carcinoma, renal cell carcinoma, small-cell lung cancer, esophageal carcinoma, and colorectal cancer.
CS1003 is a humanized IgG4 PD-1 mAb developed by CStone Pharmaceuticals (Suzhou) Co., Ltd. This study included the in vitro and in vivo characterization as well as a PK evaluation of CS1003. Either pembrolizumab or nivolumab was used as a reference in this study, and the results were equivalent to those previously reported. CS1003 binds to human, cynomolgus monkey and mouse PD-1 with high affinity and blocks the interaction of PD-1 with both PD-L1 and PD-L2 ligands. As reported, human PD-L1 also cross-reacts with mouse PD-1 [14]. R9 is a rat hybridoma of CS1003 that interacts with PD-1 in similar manner to human PD-L1 [15]. The cross-reactivity to both human and mouse PD-1 makes CS1003 to be the most straightforward and cost-effective approach for testing in mouse models. CS1003 resulted in significant TGI in both MC38-hPD-L1 colon cancer models in human PD-1 knock-in mice with comparable potency to nivolumab. Furthermore, CS1003 also showed antitumor activity in syngeneic tumor models, i.e., CT26 and MC38 models, without additional modification. These results clearly demonstrated its equivalent ability to target both human and mouse PD-1 and enhance antitumor immunity.
Although the clinical success rate of anti-PD-1 mAbs has been demonstrated to be substantially higher than that of other cancer therapies, 30%–60% of patients show no response to PD-1 blockade and are refractory to PD-1 treatment [8]. Combination therapy has become an important strategy to increase the response rate and overcome resistance. Preclinical evidence is essential to provide rationale for agent/target selection in combination strategies. Among the approved anti-PD-1 antibodies, none of them cross-reacted with mouse. Surrogate antibodies, such as RMP-1–14 from Bio X Cell, are generated and used to evaluate the efficacy in syngeneic animal models. However, due to the difference in binding epitopes, surrogate antibodies may not fully reflect the actual function and potential biology of therapeutic antibodies in humans. The cross-reaction of CS1003 with human and mouse PD-1 provides a significant advantage to test the efficacy of its combination with a variety of other antitumor agents in syngeneic mouse tumor models, which is the focus of ongoing efforts at our institution. The potential of CS1003 combined with chemotherapy or targeted therapy is now under evaluation in syngeneic models. CS1003 shows promising effects when combined with CStone’s MEK inhibitor (CS3006) and CDK4/6 inhibitor (CS3002) in the CT26 model (data not shown). Other potential combinations with new molecules from global partners are also under investigation.
CS1003 showed a linear PK profile within the dose range of 2–18 mg/kg following single intravenous administration in monkeys. The PK parameters, including CL and terminal half-life, were within the typical ranges seen for IgGs and were comparable to those of pembrolizumab and nivolumab [16, 17]. The ADA incidence was low, suggesting that CS1003 induces low immunogenicity in monkeys.
The mechanism of action of anti-PD-1 antibodies includes a complex interplay of receptor binding, target engagement and positive feedback mechanisms [18]. Receptor occupancy is an important pharmacodynamic marker for therapeutic antibody evaluation. Since nivolumab has been extensively studied in clinical trials [13, 19], we optimized the receptor occupancy assay of nivolumab to examine the PD-1 occupancy of CS1003 on circulating CD3+ T cells, including CD3+CD8+ T cells and CD3+CD4+ T cells. The RO% of CS1003 quickly reached ~100% from 24 h postdose in all single-dose groups. Compared with the PD-1 occupancy of nivolumab, which appeared to be dose-independent, with a mean peak occupancy of 85% observed at 4–24 h after one infusion in patients, the consistently high levels of PD-1 occupancy observed after CS1003 treatment indicate its potentially better efficacy.
In conclusion, CS1003 is a humanized IgG4 anti-PD-1 mAb with mouse cross-reactivity. The preclinical data are promising and provide the basis for its efficacy and safety evaluation in clinical trials.
References
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39.
Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann NY Acad Sci. 2011;1217:45–59.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42.
Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013.
Song M, Chen X, Wang L, Zhang Y. Future of anti-PD-1/PD-L1 applications: combinations with other therapeutic regimens. Chin J Cancer Res. 2018;30:157–72.
Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283:110–20.
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66.
Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–77.
Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–7.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Huang A, Peng D, Guo H, Ben Y, Zuo X, Wu F, et al. A human programmed death-ligand 1-expressing mouse tumor model for evaluating the therapeutic efficacy of anti-human PD-L1 antibodies. Sci Rep. 2017;7:42687.
Li D, Xu J, Wang Z, Gong Z, Liu J, Zheng Y, et al. Epitope mapping reveals the binding mechanism of a functional antibody cross-reactive to both human and murine programmed death 1. MAbs. 2017;9:628–37.
Merck & Co. I. Pharmacology Review of Keytruda (pembrolizumab). U.S. Food and Drug Administration website. August 22, 2014 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125514Orig1s000PharmR.pdf.
Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D. et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56.
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75.
Acknowledgements
We thank Dr. Yan-fen Teng, Quan Qiu, and Ru-mei Chen for language assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
FL, JL, KY, JZ, ZHL, LL, YWB, ZQ, YZ, BTY, and JL designed and performed the research; FL, ZHL, LL, YWB, ZQ, and XW wrote the paper; XW approved the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, F., Li, J., Yin, K. et al. CS1003, a novel human and mouse cross-reactive PD-1 monoclonal antibody for cancer therapy. Acta Pharmacol Sin 42, 142–148 (2021). https://doi.org/10.1038/s41401-020-0422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0422-6