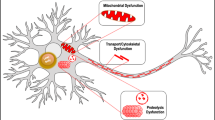
Abstract
In this review, we summarise the evidence for a role of the ribonuclease angiogenin in the pathophysiology of neurodegenerative disorders, with a specific focus on Parkinson’s disease (PD). Angiogenin is a stress-induced, secreted ribonuclease with both nuclear and cytosolic activities. Loss-of-function mutations in the angiogenin gene (ANG) have been initially discovered in familial cases of amyotrophic lateral sclerosis (ALS), however, variants in ANG have subsequently been identified in PD and Alzheimer’s disease. Delivery of angiogenin protein reduces neurodegeneration and delays disease progression in in vitro and in vivo models of ALS and in vitro models of PD. In the nucleus, angiogenin promotes ribosomal RNA transcription. Under stress conditions, angiogenin also translocates to the cytosol where it cleaves non-coding RNA into RNA fragments, in particular transfer RNAs (tRNAs). Stress-induced tRNA fragments have been proposed to have multiple cellular functions, including inhibition of ribosome biogenesis, inhibition of protein translation and inhibition of apoptosis. We will discuss recent evidence of tRNA fragment accumulation in PD, as well as their potential neuroprotective activities.
Similar content being viewed by others
Angiogenin: a unique ribonuclease with multiple cellular functions
Parkinson’s disease (PD) is characterized by the progressive degeneration and loss of dopaminergic neurons in the substantia nigra pars compacta and catecholaminergic neurons in the locus coeruleus. Loss of neurotrophic or neuroprotective support may therefore increase the likelihood of developing PD. Angiogenin is a 14.1 kDa protein that belongs to the superfamily of vertebrate secreted ribonucleases. Other members of this family include RNase 1 (pancreatic ribonuclease), RNase 2 (eosinophil-derived neurotoxin), RNase 3 (eosinophil cationic protein), RNase 4, RNase 6 (k6), RNase 7 and RNase 8 [1]. In contrast to most members of this family, angiogenin exhibits relatively low ribonuclease activity [2]. Angiogenin was initially discovered as a tumour-derived angiogenic factor in human colon adenocarcinoma cells [3]. Subsequent studies focused on its role in angiogenesis, cancer, ischaemia and infection (reviewed in [4]). As the name suggests, angiogenin stimulates endothelial cell proliferation and has been shown to be required for the angiogenic activity of vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) [5]. While VEGF and FGF-2 signal through tyrosine kinase receptors to activate protein synthesis (via stimulation of mTOR and S6 kinase pathways), angiogenin increases ribosomal RNA (rRNA) transcription in the nucleus [6]. It thereby acts synergistically with VEGF and FGF-2 to increase protein synthesis in endothelial cells and is required for their proliferation [5]. Angiogenin-induced RNA transcription also requires the ribonuclease activity of angiogenin and occurs via an epigenetic activation of the ANG promoter [6, 7]. Angiogenin has also been proposed to indirectly stimulate the PI3-kinase and Akt kinase pathways in endothelial cells and bladder cancer cells [8,9,10].
Additional cytosolic activities of angiogenin have recently emerged. Under conditions of cellular stress such as oxidative stress, disruption of proteostasis or withdrawal of trophic factors, angiogenin accumulates in cytosolic stress granules [11, 12]. Stress granules are cytoplasmic foci containing untranslated mRNA and RNA-binding proteins that are formed in response to cellular stress and function to arrest protein translation [13,14,15]. Angiogenin is also a secreted factor that can be taken up by endocytosis into the cytoplasm of target cells [16,17,18] where it may function in paracrine [16].
As angiogenin is secreted from cells, several studies investigated potential angiogenin receptors and cellular uptake mechanisms. Angiogenin has been shown to be a ligand for surface receptors of the Plexin family. Plexin-B2 was identified by Yu et al. as receptor for angiogenin in endothelial, cancer, neuronal, and normal hematopoietic and leukaemic stem and progenitor cells [19]. Plexin-B2 is expressed in the postnatal and adult nervous system particularly in subventricular zone (SZV)-derived neural stem cells [20].
Angiogenin may, however, also been taken up through additional mechanisms into other cell types. Studies from our group demonstrated that neuronally-secreted or exogenous angiogenin protein is effectively taken up into astroglia [16] and alters their secretome [21]. Indeed, astroglia are now considered an important contributor to neurodegenerative disorders including PD and amyotrophic lateral sclerosis (ALS) [22, 23]. Uptake and subsequent RNA cleavage in astrocytes have been shown to require clathrin-mediated endocytosis (CME) and dependent on heparan sulfate proteoglycans [16]. It is possible that other angiogenin-binding proteins responsible for angiogenin uptake and signalling will be identified going forward.
Regulation of angiogenin activity
The activity of angiogenin is critically regulated by an endogenous inhibitor, ribonuclease/angiogenin inhibitor 1 (RNH1) [24]. Like angiogenin, RNH1 is expressed in endothelial cells and many other cell types, including neurons and glial cells [25,26,27]. RNH1 inhibits the activity of angiogenin by binding to its catalytic triad residues Lys-40, His-13 and His-114 [28]. Under stress and anabolic conditions, RNH1 accumulates in the nucleus where it binds angiogenin and inhibits rRNA transcription to save energy [29]. Angiogenin activity is therefore a process that is determined by the relative abundance and co-localisation of both proteins in cells.
Similar to other pro-angiogenic factors such as VEGF, transcription of the ANG gene is regulated by the transcription factor hypoxia-inducible factor-1α (HIF-1α). Through this mechanisms, increased ANG expression stimulates angiogenesis in tissues that have insufficient oxygen supply [30]. The hypoxia responsive element within the ANG gene has been mapped to the consensus HIF-1α binding site 5′-RCGTG-3′ [30, 31]. HIF-1α has also been shown to be required for ANG expression in neural cells in response to hypoxia [31]. ANG expression is also positively regulated by the transcription factor hepatic nuclear factor-1α (HNF-1α) [32]. This transcription factor is involved in glucose and lipid metabolism in liver and pancreatic beta-cells.
ANG variants in ALS and PD
Angiogenin became of interest to neuroscience with the initial discovery that ANG gene variants were associated with familial and apparently sporadic cases of ALS in Scottish, Irish, English, Swedish and Northern American families [33]. As part of this study, our laboratory identified that angiogenin is expressed and enriched in motor neurons. Subsequent studies have identified ANG variants in Italian, French, German, Dutch, Belgian, Hungarian, Chinese and Indian ALS patients (Table 1). Several of the ANG variants identified were predicted and subsequently validated to affect angiogenin’s ribonuclease activity due to their proximity to the catalytic site of the protein (Table 2). Other angiogenin variants have been shown to inhibit the shuttling of angiogenin between nucleus and cytoplasm [34], or to reduce the stability of the protein [35]. Subsequent studies showed that angiogenin exerts neuroprotective activities in vitro in models of excitotoxic, hypoxic and trophic factor-withdrawal-induced injury to motor neurons and other neural cells, including dopaminergic SH-SY5Y neuroblastoma cells [31, 36, 37]. Many of the ANG variants identified were shown to have reduced neuroprotective activity compared with wild-type ANG when overexpressed at similar levels in neurons [31, 36]. However, it is currently unknown which cell types in the nervous system (including endothelial cells) are susceptible to ANG mutations.
Of note, subsequent studies also identified ANG variants in familial forms of PD [38, 39]. The two studies identified several non-synonymous ANG variants in Northern American, German, Dutch and Italian PD patients (Table 3). The frequency of PD ANG variants were highly similar in both studies (0.45%/0.47%) compared with controls (0.04%/0%). Furthermore, Van Es et al. reported similar frequency of ALS patients and PD patients carrying ANG variants (0.46%/0.45% compared with 0.04% in controls) [38]. Many of the reported PD ANG variants were predicted to impair angiogenin protein function [38]. In a more recent study, several of these variants were validated to have reduced levels of ribonuclease activity in comparison with wild-type angiogenin [40].
Interestingly, a recent study demonstrated ANG mutations in familial cases of Alzheimer’s disease (AD) [41]. Collectively, these findings point to a general role of angiogenin as a protective factor for central nervous system neurons. Of note, ang1 knock-out mice do not appear to develop neurodegeneration and ALS-, PD-, or AD-like symptoms or neuropathology in their life span [42], highlighting that aging and/or additional disease processes are required to trigger neurodegeneration. ANG can therefore be added to list of genes that regulate stress responses in neurons and are mutated and contribute to neurodegeneration in various neurological disorders (including PD), as seen in the case of TARDBP mutations, FUS mutations, C9ORF72 repeat expansions, and DJ-1 mutations [43,44,45,46].
Angiogenin delivery is neuroprotective in models of ALS and PD
The ANG mutations that have been reported in ALS and PD patients suggested a direct involvement of angiogenin in pathways leading to motoneuron degeneration or degeneration of dopaminergic neurons. We demonstrated that angiogenin protects cultured primary mouse motor neurons against ALS-associated, stress-induced cell death including excitotoxic injury by promoting and sustaining cell survival signalling through PI3-kinase/Akt kinases [36]. In further preclinical work, we demonstrated that daily, systemic (intraperitoneal) delivery of recombinant human angiogenin protein significantly increased life span and improved motor function in SOD1G93A mice, an established mouse model of ALS [36]. Cell survival signalling in motoneurons was preserved in angiogenin-treated mice. Importantly, the effect of angiogenin, when delivered post-symptom onset (from day 90 onward) on life span and disease progression, was comparable to the effect of a pre-symptom angiogenin treatment (from day 50 onward). These results suggested that angiogenin protein delivery may be beneficial in treating patients with newly diagnosed ALS.
To further validate these findings, our group performed a SOD1G93A mouse model study according to the preclinical guidelines for ALS animal studies set by the 2010 European ALS/MND group [47]. In this study, we demonstrated that systemic delivery of human angiogenin three times per week post-symptom onset (from day 90 onward) delayed motor dysfunction, significantly enhanced survival and protected against motoneuron loss and vascular network regression in the lumbar spinal cord [48].
Angiogenin may also be neuroprotective in PD. Steidinger et al. demonstrated significantly decreased levels of endogenous angiogenin in an alpha-synuclein transgenic mouse model of PD and showed that recombinant human angiogenin protected against dopaminergic neuronal cell death and inhibited caspase-3 activation in neurotoxin-induced in vitro models of PD [49]. A subsequent study by the same group found that virally-mediated overexpression of human angiogenin in the substantia nigra did not protect against dopaminergic cell loss in a neurotoxin-based mouse model of PD [50]. These findings suggest that further in vivo studies are required to explore potential neuroprotective functions in animal models of PD, in particular in genetic models.
tRNA-derived fragments (tRFs) and ‘stress-induced tRNA fragments’ (tiRNAs) in PD
During stress conditions, angiogenin accumulates in the cytosol where it cleaves non-coding RNAs, including transfer RNAs (tRNAs). Cleavage of tRNAs by angiogenin generates fragments termed ‘stress-induced tRNA fragments’ or ‘tiRNAs’ [12, 51, 52]. Cleavage of tRNAs by angiogenin occurs in their anticodon loop, a process that is highly regulated by tRNA modifications [53,54,55,56,57], so that only specific subsets of tRNA fragments are generated [58]. tiRNAs have been shown to inhibit ribosome assembly [12] and to inhibit cap-dependent protein translation via interaction with the initiation factor eIF4F [12]. Both processes may facilitate the recovery of cells during stress conditions, so that resource-consuming or error-sensitive cell functions are stalled during periods of stress. tiRNA generation has also been linked to the process of stem cell maintenance and inhibits the proliferation of hematopoietic stem/progenitor cells [42]. Due to their multiple mechanisms of action, lack of tiRNA production could be involved in the pathogenic effects of ANG variants in human disease.
tRFs in general are now considered a new class on small non-coding RNAs with multiple cellular functions, of which tiRNAs are only a subclass. tRFs have been detected in various biological systems suggesting that tRNA cleavage by angiogenin and other ribonucleases is an evolutionary conserved process. Other new functions of tRFs beyond the regulation of protein translation have also been reported. ‘SHOT-RNAs’, which are analogous to angiogenin-produced tiRNAs, were identified in hormone responsive cancer cells where they stimulate their proliferation [59]. Two studies in 2016 showed that tRFs fragments are enriched in sperm cells and delivered to the zygote at fertilization where they modified gene expression by binding to the elements in the zygote’s genome [60, 61]. Multiple ribonucleases other than angiogenin are able to generate such fingerprints, including the ribonucleases Z and Dicer [62,63,64,65,66]. The identification of tRF ‘fingerprints’ in different biological systems and disease conditions is still at its infancy, but interesting observations are now beginning to emerge. A study from our laboratory showed that specific tRFs are associated with epilepsy [67]. Small RNA sequencing analysis of plasma samples collected during video EEG monitoring of patients with focal epilepsy identified significant differences in three specific tRFs fragments compared with healthy controls. Interestingly, these tRFs are different from angiogenin-generated fragments as cleavage did not occur in the anticodon loop. These fragments were elevated in the pre-seizure period, but lower in post-seizure samples, and may represent a novel class of biomarkers indicating seizure risk in epilepsy patients.
A recent study performed in PD patients identified disease-specific tRFs in brain and biofluids [68]. Reanalyses of RNAseq data from three previous studies identified multiple differentially abundant tRFs between PD patients and healthy controls in prefrontal cortex, cerebrospinal fluid and serum. Of note, a subset of the identified tRFs successfully distinguished PD patients from controls with high sensitivity and specificity in each sample collection. Further research is required as to whether these fragments are generated by angiogenin or other ribonucleases. Collectively, these findings suggested that tRF signatures are promising candidates as non-invasive PD biomarkers.
Summary
There is a significant body of evidence suggesting that angiogenin is a stress-induced survival factor for central nervous system neurons. While it has been shown that angiogenin is able to protect against dopaminergic neuron loss in vitro, further research is required to explore its role in animal models of PD. The arrival of new animal models of PD will likely accelerate this translation, as seen in ALS models. Due to its pleiotropic mechanism of action, angiogenin may indeed be an interesting candidate for the treatment of neurodegenerative disorders. It stimulates angiogenesis in endothelial cells and promotes neuronal survival through Akt signalling and possibly through the formation of tiRNAs, thereby facilitating the recovery of stressed neurons. Moreover, tRFs generated by angiogenin and other ribonucleases may deliver novel diagnostic or prognostic tools for the management of neurodegenerative disorders. Studies are now required to explore the biological functions of these fragments in vitro and in vivo.
References
Zhang J, Dyer KD, Rosenberg HF. RNase 8, a novel RNase A superfamily ribonuclease expressed uniquely in placenta. Nucleic Acids Res. 2002;30:1169–75.
Shapiro R, Riordan JF, Vallee BL. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986;25:3527–32.
Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–6.
Sheng J, Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim Biophys Sin. 2016;48:399–410.
Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–56.
Xu ZP, Tsuji T, Riordan JF, Hu GF. The nuclear function of angiogenin in endothelial cells is related to rRNA production. Biochem Biophys Res Commun. 2002;294:287–92.
Sheng J, Yu W, Gao X, Xu Z, Hu GF. Angiogenin stimulates ribosomal RNA transcription by epigenetic activation of the ribosomal DNA promoter. J Cell Physiol. 2014;229:521–9.
Kim HM, Kang DK, Kim HY, Kang SS, Chang SI. Angiogenin-induced protein kinase B/Akt activation is necessary for angiogenesis but is independent of nuclear translocation of angiogenin in HUVE cells. Biochem Biophys Res Commun. 2007;352:509–13.
Trouillon R, Kang DK, Park H, Chang SI, O’Hare D. Angiogenin induces nitric oxide synthesis in endothelial cells through PI-3 and Akt kinases. Biochemistry. 2010;49:3282–8.
Peng Y, Li L, Huang M, Duan C, Zhang L, Chen J. Angiogenin interacts with ribonuclease inhibitor regulating PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell Signal. 2014;26:2782–92.
Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, et al. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285:10959–68.
Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–23.
Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–42.
Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, et al. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–68.
Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210.
Skorupa A, King MA, Aparicio IM, Dussmann H, Coughlan K, Breen B, et al. Motoneurons secrete angiogenin to induce RNA cleavage in astroglia. J Neurosci. 2012;32:5024–38.
Ferguson R, Subramanian V. The cellular uptake of angiogenin, an angiogenic and neurotrophic factor is through multiple pathways and largely dynamin independent. PLoS ONE 2018;13:e0193302.
Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci USA. 1994;91:1677–81.
Yu W, Goncalves KA, Li S, Kishikawa H, Sun G, Yang H, et al. Plexin-B2 mediates physiologic and pathologic functions of angiogenin. Cell 2017;171:849–64 e25.
Saha B, Ypsilanti AR, Boutin C, Cremer H, Chedotal A. Plexin-B2 regulates the proliferation and migration of neuroblasts in the postnatal and adult subventricular zone. J Neurosci. 2012;32:16892–905.
Skorupa A, Urbach S, Vigy O, King MA, Chaumont-Dubel S, Prehn JHM, et al. Angiogenin induces modifications in the astrocyte secretome: relevance to amyotrophic lateral sclerosis. J Proteom. 2013;91:274–85.
Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–72.
Hinkle JT, Dawson VL, Dawson TM. The A1 astrocyte paradigm: new avenues for pharmacological intervention in neurodegeneration. Mov Disord. 2019;34:959–69.
Shapiro R, Vallee BL. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci USA. 1987;84:2238–41.
Dickson KA, Haigis MC, Raines RT. Ribonuclease inhibitor: structure and function. Prog Nucleic Acid Res Mol Biol. 2005;80:349–74.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Human Protein Atlas. Version 19. RNH1; 2005. https://www.proteinatlas.org/ENSG00000023191-RNH1/tissue.
Lee FS, Vallee BL. Binding of placental ribonuclease inhibitor to the active site of angiogenin. Biochemistry. 1989;28:3556–61.
Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, et al. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci. 2013;126:4308–19.
Lai K, Luo C, Zhang X, Ye P, Zhang Y, He J, et al. Regulation of angiogenin expression and epithelial-mesenchymal transition by HIF-1alpha signaling in hypoxic retinal pigment epithelial cells. Biochim Biophys Acta. 2016;1862:1594–607.
Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, et al. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16:1238–47.
Dyer KD, Rosenberg HF. The mouse RNase 4 and RNase 5/ang 1 locus utilizes dual promoters for tissue-specific expression. Nucleic Acids Res. 2005;33:1077–86.
Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–3.
Wu D, Yu W, Kishikawa H, Folkerth RD, Iafrate AJ, Shen Y, et al. Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis. Ann Neurol. 2007;62:609–17.
Crabtree B, Thiyagarajan N, Prior SH, Wilson P, Iyer S, Ferns T, et al. Characterization of human angiogenin variants implicated in amyotrophic lateral sclerosis. Biochemistry. 2007;46:11810–8.
Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, et al. Control of Motoneuron Survival by Angiogenin. J Neurosci. 2008;28:14056–61.
Subramanian V, Crabtree B, Acharya KR. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum Mol Genet. 2008;17:130–49.
van Es MA, Schelhaas HJ, van Vught PW, Ticozzi N, Andersen PM, Groen EJ, et al. Angiogenin variants in Parkinson disease and amyotrophic lateral sclerosis. Ann Neurol 2011;70:964–73.
Rayaprolu S, Soto-Ortolaza A, Rademakers R, Uitti RJ, Wszolek ZK, Ross OA. Angiogenin variation and Parkinson disease. Ann Neurol. 2012;71:725–7. author reply. 7-8
Bradshaw WJ, Rehman S, Pham TT, Thiyagarajan N, Lee RL, Subramanian V, et al. Structural insights into human angiogenin variants implicated in Parkinson’s disease and Amyotrophic Lateral Sclerosis. Sci Rep. 2017;7:41996.
Gagliardi S, Davin A, Bini P, Sinforiani E, Poloni TE, Polito L, et al. A novel nonsense angiogenin mutation is associated with Alzheimer disease. Alzheimer Dis Assoc Disord. 2019;33:163–5.
Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell 2016;166:894–906.
Taylor JP, Brown RH Jr., Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206.
Cannas A, Borghero G, Floris GL, Solla P, Chio A, Traynor BJ, et al. The p.A382T TARDBP gene mutation in Sardinian patients affected by Parkinson’s disease and other degenerative parkinsonisms. Neurogenetics. 2013;14:161–6.
Annesi G, Savettieri G, Pugliese P, D’Amelio M, Tarantino P, Ragonese P, et al. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol. 2005;58:803–7.
Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, et al. C9orf72 repeat expansions are a rare genetic cause of parkinsonism. Brain. 2013;136:385–91.
Ludolph AC, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler JP, et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Scler Frontotemporal Degener. 2010;11:38–45.
Crivello M, O’Riordan SL, Woods I, Cannon S, Halang L, Coughlan KS, et al. Pleiotropic activity of systemically delivered angiogenin in the SOD1(G93A) mouse model. Neuropharmacology. 2018;133:503–11.
Steidinger TU, Standaert DG, Yacoubian TA. A neuroprotective role for angiogenin in models of Parkinson’s disease. J Neurochem. 2011;116:334–41.
Steidinger TU, Slone SR, Ding H, Standaert DG, Yacoubian TA. Angiogenin in Parkinson disease models: role of Akt phosphorylation and evaluation of AAV-mediated angiogenin expression in MPTP treated mice. PLoS ONE 2013;8:e56092.
Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, et al. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–42.
Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42.
Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5.
Wang X, Matuszek Z, Huang Y, Parisien M, Dai Q, Clark W, et al. Queuosine modification protects cognate tRNAs against ribonuclease cleavage. RNA. 2018;24:1305–13.
Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–45.
Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018;20:535–40.
Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014;33:2020–39.
Su Z, Kuscu C, Malik A, Shibata E, Dutta A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J Biol Chem. 2019;294:16930–41.
Honda S, Kirino Y. SHOT-RNAs: a novel class of tRNA-derived functional RNAs expressed in hormone-dependent cancers. Mol Cell Oncol. 2016;3:e1079672.
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400.
Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–6.
Cole C, Sobala A, Lu C, Thatcher SR, Bowman A, Brown JW, et al. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–60.
Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–85.
Li Z, Ender C, Meister G, Moore PS, Chang Y, John B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012;40:6787–99.
Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009;23:2639–49.
Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA 2010;16:673–95.
Hogg MC, Raoof R, El Naggar H, Monsefi N, Delanty N, O’Brien DF, et al. Elevation in plasma tRNA fragments precede seizures in human epilepsy. J Clin Invest. 2019;129:2946–51.
Magee R, Londin E, Rigoutsos I. TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson’s disease. Parkinsonism Relat Disord. 2019;65:203–9.
Tripolszki K, Danis J, Padhi AK, Gomes J, Bozo R, Nagy ZF, et al. Angiogenin mutations in Hungarian patients with amyotrophic lateral sclerosis: clinical, genetic, computational, and functional analyses. Brain Behav. 2019;9:e01293.
Gellera C, Colombrita C, Ticozzi N, Castellotti B, Bragato C, Ratti A, et al. Identification of new ANG gene mutations in a large cohort of Italian patients with amyotrophic lateral sclerosis. Neurogenetics. 2008;9:33–40.
Fernandez-Santiago R, Hoenig S, Lichtner P, Sperfeld AD, Sharma M, Berg D, et al. Identification of novel Angiogenin (ANG) gene missense variants in German patients with amyotrophic lateral sclerosis. J Neurol. 2009;256:1337–42.
van Es MA, Diekstra FP, Veldink JH, Baas F, Bourque PR, Schelhaas HJ, et al. A case of ALS-FTD in a large FALS pedigree with a K17I ANG mutation. Neurology. 2009;72:287–8.
van Blitterswijk M, van Es MA, Hennekam EA, Dooijes D, van Rheenen W, Medic J, et al. Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21:3776–84.
Seilhean D, Cazeneuve C, Thuries V, Russaouen O, Millecamps S, Salachas F, et al. Accumulation of TDP-43 and alpha-actin in an amyotrophic lateral sclerosis patient with the K17I ANG mutation. Acta Neuropathol. 2009;118:561–73.
Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J Med Genet. 2010;47:554–60.
Paubel A, Violette J, Amy M, Praline J, Meininger V, Camu W, et al. Mutations of the ANG gene in French patients with sporadic amyotrophic lateral sclerosis. Arch Neurol. 2008;65:1333–6.
Conforti FL, Sprovieri T, Mazzei R, Ungaro C, La Bella V, Tessitore A, et al. A novel Angiogenin gene mutation in a sporadic patient with amyotrophic lateral sclerosis from southern Italy. Neuromuscul Disord. 2008;18:68–70.
Zou ZY, Wang XN, Liu MS, Sun Q, Li XG, Cui LY, et al. Identification of a novel missense mutation in angiogenin in a Chinese amyotrophic lateral sclerosis cohort. Amyotroph Lateral Scler Frontotemporal Degener. 2012;13:270–5.
Luigetti M, Lattante S, Zollino M, Conte A, Marangi G, Del Grande A, et al. SOD1 G93D sporadic amyotrophic lateral sclerosis (SALS) patient with rapid progression and concomitant novel ANG variant. Neurobiol Aging. 2011;32:1924 e15–8.
Narain P, Padhi AK, Dave U, Mishra D, Bhatia R, Vivekanandan P, et al. Identification and characterization of novel and rare susceptible variants in Indian amyotrophic lateral sclerosis patients. Neurogenetics. 2019;20:197–208.
Thiyagarajan N, Ferguson R, Subramanian V, Acharya KR. Structural and molecular insights into the mechanism of action of human angiogenin-ALS variants in neurons. Nat Commun. 2012;3:1121.
Acknowledgements
This publication has emanated from research supported in part by a research grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3948 and co-funded under the European Regional Development Fund and by FutureNeuro industry partners and by the Joint Programme in Neurodegeneration Research (JPND) project RNA-NEURO supported by SFI (17/JPND/3455). EJ is supported by a StAR International PhD Scholarship from the Royal College of Surgeons in Ireland.
Author information
Authors and Affiliations
Contributions
JHMP and EJ wrote the paper.
Corresponding author
Ethics declarations
Competing interests
JHMP is a beneficiary of a patent relating to the use of angiogenin as a diagnostic and therapeutic for ALS and other neurodegenerative disorders. EJ declares no competing interest.
Rights and permissions
About this article
Cite this article
Prehn, J.H.M., Jirström, E. Angiogenin and tRNA fragments in Parkinson’s disease and neurodegeneration. Acta Pharmacol Sin 41, 442–446 (2020). https://doi.org/10.1038/s41401-020-0375-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0375-9
Keywords
This article is cited by
-
Roles and regulation of tRNA-derived small RNAs in animals
Nature Reviews Molecular Cell Biology (2024)
-
Translation dysregulation in neurodegenerative diseases: a focus on ALS
Molecular Neurodegeneration (2023)
-
RNA methyltransferase NSun2 deficiency promotes neurodegeneration through epitranscriptomic regulation of tau phosphorylation
Acta Neuropathologica (2023)
-
The role of noncoding RNAs in Parkinson’s disease: biomarkers and associations with pathogenic pathways
Journal of Biomedical Science (2021)
-
Deciphering the tRNA-derived small RNAs: origin, development, and future
Cell Death & Disease (2021)