Abstract
Recent studies show that the expression of CCND1, a key factor in cell cycle control, is increased following the progress and deteriotation of glioma and predicts poor outcomes. On the other hand, dysregulated deubiquitinase USP10 also predicts poor prognosis for patients with glioblastoma (GBM). In the present study, we investigated the interplay between CCND1 protein and USP10 in GBM cells. We showed that the expression of CCND1 was significantly higher in both GBM tissues and GBM-derived stem cells. USP10 interacted with CCND1 and prevented its K48- but not K63-linked polyubiquitination in GBM U251 and HS683 cells, which led to increased CCND1 stability. Consistent with the action of USP10 on CCND1, knockdown of USP10 by single-guided RNA downregulated CCND1 and caused GBM cell cycle arrest at the G1 phase and induced GBM cell apoptosis. To implement this finding in the treatment of GBMs, we screened a natural product library and found that acevaltrate (AVT), an active component derived from the herbal plant Valeriana jatamansi Jones was strikingly potent to induce GBM cell apoptosis, which was confirmed by the Annexin V staining and activation of the apoptotic signals. Furthermore, we revealed that AVT concentration-dependently suppressed USP10-mediated deubiquitination on CCND1 therefore inducing CCND1 protein degradation. Collectively, the present study demonstrates that the USP10/CCND1 axis could be a promising therapeutic target for patients with GBMs.
Similar content being viewed by others
Introduction
Glioblastoma (GBM) is a fast-growing, very aggressive, and largely resistant brain tumor. Survival periods have not been significantly improved for decades despite the introduction of various novel effective therapies [1]. The average survival period is 9–12 months, and the survival over 5 years is <2%. The current modalities for GBM treatment include surgery, radiation, and chemotherapy, of which surgery can be applied for most GBM patients; however, recurrence and resistance are frequently seen even after successful neurosurgical resection [2]. Therefore, radiation and/or chemotherapy directed toward specific molecular events is widely used after surgery or in patients who do not undergo surgery. Understanding the broad spectrum of events associated with refractory/recurrent GBM is essential and urgent for the successful treatment of GBM.
Cyclin D1 (CCND1), a member of the D-type cyclin proteins (D-cyclins), is a key element in the control of cell cycle progression [3]. During the transition of the cell cycle from G1 to S phase, CCND1 binds to and activates cyclin-dependent kinase 4/6 (CDK4/6) to trigger phosphorylation of the retinoblastoma protein (Rb), thus releasing the transcription factor E2F and initiating DNA synthesis [3]. D-cyclins harbor a specific structure called the PEST domain that is characteristic of proteins that are rapidly turned over after they exercise their functions in the G1-to-S phase transition of the cell cycle [4]. Therefore, D-cyclins are normally synthesized de novo at the appropriate time during the cell cycle transition. However, D-cyclins are frequently overexpressed in many cancer types, including multiple myeloma and mantle cell lymphoma [5]. A recent study revealed that the expression of CCND1 is increased following the progression and deterioration of glioma and that its upregulation predicts poor outcomes [6]. CCND1 and CDK4 may be required for glioma development, and the two proteins mediate the development of neurologically destructive oligodendroglioma. Therefore, genetic loss of CCND1 impedes oligodendroglioma progression to higher stages of malignancy [7]. However, how the CCND1 protein is modulated in GBM is largely unknown.
Because CCND1 harbors the PEST domain for rapid degradation, a critical factor for its action in the modulation of cell cycle progression and cell proliferation [8], investigation of CCND1 degradation is important for enabling control of cancer cell proliferation. The quick turnover of CCND1 is processed via the ubiquitin-proteasomal pathway, and the Skp1-Cul1-F-box (SCF) family of E3 ubiquitin ligases, including ROC1–cullin and FBX4, might mediate CCND1 ubiquitination [9]. In addition, some deubiquitinases, including USP2a [10] and USP22 [11], have been reported to stabilize CCND1 and promote its activity. When USP2a is inhibited by the small-molecule compound lithocholic acid hydroxyamide, CCND1 undergoes degradation, resulting in defects in cell cycle progression and suppression of cancer cell growth [12]. We identified D-cyclin deubiquitinases by mass spectrometry and found that ubiquitin-specific protease 10 (USP10) interacts with cyclin D1. In the present study, we found that USP10 stabilizes CCND1 by preventing its K48-linked polyubiquitination in GBM. Moreover, we found that the natural product acevaltrate (AVT) can target USP10/CCND1 and induce GBM cell apoptosis.
Materials and methods
Cells and cell culture
Human embryonic kidney cells (HEK293T) and GBM cell lines (U251, HS683) were grown in Dulbecco’s modified Eagle’s medium. The U251 cell line was isolated from a malignant GBM through an explant technique [13], while HS683 is a typical GBM cell line established from explant cultures of a glioma taken from the left temporal lobe of a 76-year-old Caucasian male [14]. All cells were cultured in 10% fetal bovine serum (ExCell Bio, Inc., Shanghai, China) with appropriate antibiotics.
Plasmids and antibodies
USP10 and CCND1 plasmids were subcloned into a pcDNA3.1 vector carrying a Flag or Myc tag (Invitrogen, Carlsbad, USA). USP10 and control siRNAs were provided by RiboBio, Inc. (Guangzhou, China). The siRNAs were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The antibodies used for immunoblotting were as follows: anti-Ub was obtained from Santa Cruz Biotechnology Co., Ltd. (Santa Cruz, CA, USA); anti-GAPDH was obtained from Proteintech Group, Inc. (Wuhan, China); anti-HA, anti-Myc and anti-Flag were obtained from MBL Biotech Co., Ltd. (Beijing, China); and anti-poly ADP-ribose polymerase (PARP), anti-USP10, anti-Bcl-2, anti-Mcl-1, anti-p53, anti-K48 and anti-K63 were purchased from Cell Signaling Technologies Co., Ltd. (Danvers, MA, USA).
Immunoblot (IB) analysis
Whole-cell lysates were prepared from cells of interest by using cell lysis buffer. The cell lysates were then clarified by centrifugation at 10,000 × g for 30 min, and the protein concentrations in the supernatants were determined with a BCA protein assay kit (Beyotime Institute of Biotechnology, Haimen, China). The proteins were fractionated by 10% SDS-PAGE and then transferred to PVDF membranes that were then probed with specific antibodies of interest. Afterwards, the membranes were further incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit IgG secondary antibodies for 1 h at room temperature. Detection was performed on an imaging system (Tanon Science & Technology Co., Ltd., Shanghai, China) after reaction with an enhanced chemiluminescence reagent (Beyotime).
Immunoprecipitation (IP)
Cell lysates from cells of interest were prepared in RIPA buffer. After clarification and concentration determination, equal amounts of total proteins were incubated with specific antibodies overnight at 4 °C and then incubated with Protein A + G beads for 1 h at room temperature. After washing, the protein-containing beads were collected and boiled in 2× SDS loading dye before being subjected to IB assays.
Reverse transcription-PCR (RT-PCR)
Total RNA was extracted and purified from 3 × 106 cells using TRIzol® reagent (Invitrogen) following the manufacturer’s instructions. The RNA was then subjected to reverse transcription into first-strand complementary DNA using a cDNA Synthesis Kit (Beyotime). PCR amplification was performed with the following primers: for USP10, 5′-GTGACACTTTGCCGAGAAC-3′ (forward) and 5′-TCCGCCTCCACATTAGAAC-3′ (reverse); for CCND1, 5′-AAAATGCCAGAGGCGGATGA-3′ (forward) and 5′-GAAAGTGCGTTGTGCGGTAG-3′ (reverse); and for GAPDH, 5′-AATCCCATCACCATCTTCC-3′ (forward) and 5′- CATCACGCCACAGTTTCC-3′ (reverse). The RT-PCR products were visualized with GoldView staining agent (Transgen, Beijing, China) following electrophoresis on 2% agarose gels.
USP10 lentivirus construction
To generate a USP10-expressing lentivirus, a cDNA fragment of USP10 was generated by using the following primers: 5′-CCGCTCGAGATGGCCCTCCACAGCCC-3′ (forward) and 5′-TGCTTCGAATTACAGCAGGTCCACTCGG-3′ (reverse). The USP10 cDNA was inserted into a pLVX-AcGFP lentiviral vector (Clontech) between the XhoI and BstBI sites. USP10 knockdown was achieved by using the CRISPR/Cas9 gene editing technique with the following primers: 5′-CACCGGACTCCTCGATCTTCAGTTG-3′ and 5′-AAACCAACTGAAGATCGAGGAGTCC-3′. The viral particles were prepared according to the manufacturer’s instructions (Shanghai GeneChem Co., Ltd.). The plasmids were cotransfected into HEK293T cells with the calcium precipitation method. Viruses were obtained 72 h later from the transfected cells.
Cycloheximide (CHX) chase assay
After being transfected with plasmids of interest for 24 h, HEK293T cells were treated with CHX (100 μg/mL, Sigma-Aldrich) for the indicated periods as needed. Cell lysates were then prepared for SDS-PAGE and IB assays with specific antibodies.
Screening of inducers of GBM cell death
Pre-plated U251 and HS683 cells in 96-well plates were incubated with each compound (10 µM) from the Natural Product Collection provided by Target Molecule Corp., Wellesley Hills, MA. Twenty-four hours later, cell viability was evaluated by MTT assay [15]. Compounds that resulted in <75% cell viability in both cell lines were singled out for further study.
Cell viability, proliferation, and apoptosis assays
GBM cells were seeded in 96-well plates and treated with AVT (MedChemExpress LLC, Monmouth Junction, USA) for 48 h before being subjected to MTT assay. To determine apoptosis, cells treated with AVT were stained with Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and propidium iodide (PI) according to the manufacturer’s instructions (MultiSciences Biotech Co., Ltd., Hangzhou, China). The stained cells were analyzed on a flow cytometer (FACSCalibur®, Becton Dickinson). To measure cell proliferation, cells treated with AVT were stained with a Cell-LightTM EdU Apollo567 In Vitro Kit (RiboBio, Guangzhou, China) and further analyzed by fluorescence microscopy.
Statistical analyses
Statistical differences between the control and the experimental groups were analyzed by Student’s t test. All statistical tests were two-sided, and a P value < 0.05 was considered to indicate statistical significance.
Results
CCND1 is highly expressed in GBM
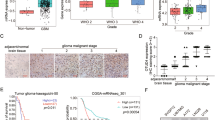
CCND1 is frequently dysregulated in many cancers, including multiple myeloma and mantle cell lymphoma, due to chromosomal translocations and other undefined mechanisms [16]. CCND1 dysregulation is also found in some low-grade gliomas, e.g., oligodendrogliomas [7], but it remains unknown whether it occurs in GBM, the most advanced form of glioma. To investigate this possibility, we first evaluated CCND1 expression levels in GBM based on public databases at www.oncomine.org. CCND1 was found to be highly expressed in GBM tissues compared with normal brain tissues (Fig. 1a), and this difference was most significant in stem cells [17]. As shown in Fig. 1b, the levels of CCND1 in GBM-derived stem cells were eightfold greater than those in normal brain stem cells [18]. These findings were consistent with the results of an analysis of gene expression in GBM patients with good and poor prognoses, which indicated that CCND1 expression levels were 3.2-fold higher in patients with poor prognosis than in those with good prognosis [18].
CCND1 interacts with USP10 in GBM
Given that CCND1 stability is important for cancer cell proliferation, some ubiquitin ligases and deubiquitinases have been identified to modulate CCND1 ubiquitination and degradation in proteasomes. We wondered whether any other deubiquitinases can modulate CCND1 stability. In the analysis of CCND1 degradation process, we found that the deubiquitinase USP10 was able to interact with CCND1. As shown in Fig. 2a, b, when USP10 and CCND1 plasmids were co-transfected into HEK293T cells, reciprocal immunoprecipitation assays showed that CCND1 (USP10) was present in the immunoprecipitates of USP10 (CCND1). These findings were also recapitulated in GBM cell lines (Fig. 2c, d). Therefore, USP10 and CCND1 interact with each other in GBM cells.
a, b HEK293T cells were co-transfected with Myc-USP10 and Flag-CCND1 for 48 h, and then cell lysates were prepared. The lysates were subjected to reciprocal immunoprecipitation (IP)/immunoblot (IB) analyses with specific antibodies, as indicated. c, d GBM cell lines were incubated with anti-USP10 or anti-CCND1 antibodies overnight and then subjected to IB with specific antibodies as indicated. Total cell lysates (TCLs) were subjected to IB as loading controls.
USP10 prevents CCND1 from undergoing K48-linked polyubiquitination in GBM cells
Because USP10 is a ubiquitin-specific protease and is able to hydrolyze ubiquitin molecules from substrate proteins, we next wondered whether USP10 is able to prevent CCND1 ubiquitination. To assess this possibility, we co-transfected USP10, Ub, and CCND1 plasmids into HEK293T cells and performed IP/IB assays. As shown in Fig. 3a, CCND1 could be heavily ubiquitinated; however, in the presence of USP10, the polyubiquitination levels were markedly reduced. This finding was also recapitulated in GBM cells after enforced expression of USP10. As shown in Fig. 3b, USP10 significantly decreased the polyubiquitination levels in both U251 and HS683 cells. It has been reported that ubiquitination can occur at any lysine residues in ubiquitin proteins and that each specific ubiquitination type will lead to a unique fate for the target proteins. The two most prominent types are the K48- and K63-linked polyubiquitination types. Therefore, we generated K48- and K63-intact ubiquitin plasmids and transfected them into HEK293T cells with or without USP10. Subsequent analyses revealed that CCND1 was mainly ubiquitinated in a K48-linked manner and that this modification could be strikingly ablated by USP10 (Fig. 3c). Moreover, this finding was also confirmed in GBM cells. As shown in Fig. 3d, upon overexpression of USP10, K48-linked ubiquitination, but not K63-linked ubiquitination, was markedly reduced. Therefore, USP10 interacts with CCND1 and prevents its K48-linked polyubiquitination.
a HEK293T cells were transfected with Flag-USP10, Myc-CCND1, and HA-Ub plasmids for 48 h and then subjected to IP/IB assays with specific antibodies as indicated. b U251 and HS683 cells were infected with lentiviruses expressing USP10 for 72 h. Then, cell lysates were prepared, and IP/IB assays were performed. c HEK293T cells were transfected with Flag-USP10, Myc-CCND1, K48-HA-Ub and K63-HA-Ub plasmids for 48 h, and then IP/IB assays were performed with specific antibodies as indicated. d U251 cells were infected with lentiviruses expressing USP10. Forty-eight hours later, cells were collected for lysate preparation and IP/IB assays as indicated.
USP10 stabilizes CCND1 in GBM cells
The above findings showed that USP10 prevents CCND1 from K48-linked polyubiquitination, a hallmark of protein degradation in proteasomes; therefore, we wondered whether USP10 can stabilize CCND1. To this end, USP10 was overexpressed or knocked down in HEK293T cells, and CCND1 abundance was measured by IB analysis. As shown in Fig. 4a, b, overexpression of USP10 increased CCND1 protein levels in a concentration-dependent manner (Fig. 4a), while knockdown decreased CCND1 protein levels (Fig. 4b). To verify this finding in GBM, USP10 was overexpressed in U251 and HS683 cells, and the subsequent assay showed that CCND1 was increased in both cell lines (Fig. 4c). Moreover, a CHX chase assay in which de novo protein synthesis was inhibited by cycloheximide showed that the half-life of CCND1 was shorter than 1 h; however, when USP10 was introduced, CCND1 stability was strikingly increased, and its half-life was extended to ~4 h (Fig. 4d, e). This finding was also confirmed in U251 cells (Fig. 4f). Therefore, USP10 stabilizes CCND1 by interacting with CCND1 and preventing its K48-linked polyubiquitination.
a HEK293T cells were transfected with Myc-USP10 and Myc-CCND1 for 48 h, after which IB assays were performed with the indicated antibodies. b USP10 was knocked down in HEK293T cells with siUSP10 for 48 h, and then an IB assay was performed. c U251 and HS683 cells were infected with lentiviruses expressing USP10 for 96 h, and then cell lysates were prepared. The lysates were subjected to IB assay as indicated. d HEK293T cells were transfected with Flag-USP10 and Myc-CCND1 for 24 h and then treated with CHX for 0–4 h. The cell lysates were used for IB analysis to measure the protein levels of USP10 and CCND1. e The intensities of the CCND1 protein bands from (d) compared to those of β-tubulin bands were analyzed with ImageJ® software. f U251 cells were transfected with Myc-USP10. Ninety-six hours later, the cells were treated with CHX for 0–4 h. The cell lysates were used for IB analysis to measure the protein levels of USP10 and CCND1.
USP10 promotes GBM cell proliferation
Several Dubs, such as USP39 [19] and USP48 [20], have been found in GBM cells and contribute to GBM growth and progression. We next wondered whether inhibition of USP10 can lead to GBM cell death. To explore this possibility, after USP10 knockdown, U251 and HS683 cells were subjected to apoptosis analysis using Annexin V staining and flow cytometric assays. As shown in Fig. 5a, when USP10 was knocked down by CRISPR/CAS-9, CCND1 expression was markedly reduced. Accordingly, the fractions of Annexin V-positive cells were strikingly increased in both cell lines (Fig. 5b), indicating that knockdown of USP10 resulted in GBM cell apoptosis. Because CCND1 is a key factor controlling cell cycle progression from G1 to S phase, we next measured the cell cycle in both U251 and HS683 cells after USP10 knockout. As expected, both cell lines were arrested in the G1 phase following USP10 knockdown (Fig. 5c), which was consistent with CCND1 degradation, as shown in Fig. 5a. Finally, USP10 was found to promote GBM cell proliferation. As shown in Fig. 5d, when cells were infected with a USP10-expressing lentivirus, increased DNA incorporation of EdU, an analog of uridine, was observed during active DNA synthesis, an indicator of cell proliferation. Therefore, USP10 promotes GBM cell proliferation, while inhibition of USP10 leads to GBM cell cycle arrest and apoptosis.
a, b Knockdown was conducted in glioma cell lines by using CRISPR/Cas9 gene editing for 48 h, after which an IB assay (a), Annexin V/PI staining and flow cytometric analyses (b) were performed. c After knockdown of USP10 by CRISPR/CAS9, U251 and HS683 cells were collected for treatment with ice-cold 75% ethanol overnight. After washing, the cells were subjected to PI staining and flow cytometric analysis. d U251 and HS683 cells were incubated with EdU for 12 h. The cells were then fixed and permeabilized before being detected on a fluorescence microscope.
The natural product AVT induces GBM cell apoptosis
Given that inhibition of USP10 induces GBM cell death, we wondered whether any natural products are able to induce GBM cell death by targeting USP10. To investigate, we screened the natural product library composed of ~1400 compounds. The cell viability assay showed that AVT, one of the most active valpotriate components derived from the herbal plant Valeriana jatamansi Jones [21], displayed potent GBM cell death-inducing activity in both cell lines (Fig. 6a). To confirm this finding, GBM cells were treated with increasing concentrations of AVT. As shown in Fig. 6b, AVT at 4 µM induced ~40% GBM cell death within 24 h as determined by Annexin V staining, and this effect was concentration-dependent. Moreover, cell viability was also decreased in a concentration-dependent manner (Fig. 6c). The occurrence of apoptosis was also confirmed by PARP and Caspase-3 cleavage (Fig. 6d), the hallmark of apoptosis. In accordance, the expression of pro-survival proteins, including Bcl-2 and Mcl-1, was downregulated in a concentration-dependent manner (Fig. 6e).Therefore, the natural product AVT induces GBM cell death.
a U251 and HS683 cells were separately plated in 96-well plates and then incubated with each compound from a natural product collection for 24 h. Cell viability was evaluated by MTT assay. AVT is indicated by the arrow in both cell line assays. b U251 and HS683 cells were treated with AVT for 24 h and then subjected to Annexin V/PI staining and flow cytometric analyses. c Glioma cell lines were treated with AVT for 24 h and then subjected to an MTT assay. d U251 and HS683 cells were treated with AVT for 24 h before being lysed and subjected to IB assays against the PARP and Caspase-3 proteins. e The cell lysates from (d) were subjected to IB assays against pro-survival proteins.
AVT promotes CCND1 degradation by inhibiting USP10 deubiquitinating function
Because AVT is able to induce GBM cell apoptosis, we wondered whether it can alter USP10 function in association with CCND1 stability. To assess this possibility, GBM cells were treated with AVT and then subjected to IB assays. As shown in Fig. 7a and c, AVT downregulated CCND1 protein expression in a concentration-dependent manner but did not significantly alter CCND1 mRNA expression (Fig. 7b, d). This finding suggests that AVT might interfere with USP10 activity related to CCND1 ubiquitination. Therefore, we next measured CCND1 ubiquitination levels in the presence of both USP10 and AVT. This assay was first performed in HEK293T cells. As shown in Fig. 7e, USP10 reduced CCND1 ubiquitination levels, but the levels were restored by AVT. Moreover, the effects of AVT on CCND1 were markedly reduced by overexpression of USP10 (Fig. 7e). Furthermore, AVT strikingly increased CCND1 polyubiquitination in both GBM cell lines while decreasing CCND1 levels (Fig. 7f). Therefore, AVT induces GBM cell death by suppressing the USP10/CCND1 axis.
a, b Glioma cells were treated with AVT for 24 h and then subjected to IB (a) or RT-PCR (b) analysis to evaluate the expression of specific genes. c–d The protein and mRNA levels of USP10 and CCND1 from (a) and (b) were analyzed by densitometry of each band. e USP10 and CCND1 plasmids were co-transfected into HEK293T cells for 12 h, after which the cells were treated with AVT. Twenty-four hours later, cell lysates were prepared and subjected to an IP/IB assay. f Glioma cell lines were treated with AVT for 24 h, after which cell lysates were prepared. IP/IB assays were performed as indicated.
Discussion
The experiments in this study revealed that USP10 interacts with and prevents K48-linked polyubiquitination of CCND1, therefore stabilizing CCND1, and that inhibition of USP10/CCND1 leads to GBM cell apoptosis.
USP10 is a ubiquitin-specific protease that cleaves conjugated ubiquitin molecules from substrate proteins. USP10 has been found to be expressed in many cancer types, including non-small cell lung cancer, hepatocellular carcinoma, prostate cancer, leukemia and others. USP10 has been demonstrated to inhibit lung cancer cell growth and invasion by stabilizing PTEN [22] while inhibiting p53 signaling and contributes to the poor outcomes of patients with prostate cancer [23]. USP10 also promotes colon cancer proliferation by deubiquitinating Musashi-2 [24]. One study has revealed that USP10 expression is approximately twofold higher in GBM brain tumor tissues than in normal brain tissues, as determined by real-time quantitative low-density array, and suggested that USP10 expression is highly associated with poor clinical outcomes [25]. The present study reveals that USP10 targets CCND1 and promotes GBM survival.
CCND1, a key factor promoting cancer cell cycle progression, survival and proliferation, is a typical substrate of the ubiquitin-proteasome pathway. Specific ubiquitin ligases for CCND1 have been identified, including FBX4 [26]. Some deubiquitinases, including USP2a and USP22 [11], have also been identified as modulators of CCND1 stability [10], but their roles in GBM are not known. The present study adds USP10 as a novel deubiquitinase for CCND1. As demonstrated in the study, USP10 can bind to CCND1 and prevent it from undergoing K48-linked polyubiquitination, therefore increasing CCND1 stability and promoting GBM cell proliferation and survival. Accordingly, suppression of USP10 leads to GBM cell apoptosis, which lays a solid basis for the treatment of GBM via targeting of USP10/CCND1.
Moreover, we identified that AVT, a traditional herbal product, induces GBM cell apoptosis by suppressing USP10/CCND1 expression. AVT is extracted from the root of an herbal plant in Valeriana, a genus of flowering plants in the family Caprifoliaceae [27]. Valeriana grows across the world, including in Northern America and Europe and especially in many countries in Asia. In general, Valeriana is well tolerated, with side effects such as dizziness, hangover-like symptoms or headache reported occasionally [28]; therefore, active components from Valeriana roots are traditionally used as herbal remedies for many neurological conditions, including insomnia, anxiety, stress, depression, attention deficit disorder, chronic fatigue syndrome, tremors, and epilepsy, especially in Asia [29]. The present study added that AVT is effective in inhibiting USP10 activity, therefore leading to CCND1 polyubiquitination accumulation that results in CCND1 degradation. This finding is further supported by the measurement of CCND1 mRNA, which indicated that AVT downregulated CCND1 protein expression but not CCND1 mRNA expression. Because CCND1 is a key switch in cell cycle progression, cancer cell proliferation and survival, inhibition of CCND1 has been demonstrated to induce cancer cell apoptosis [30]. Consistent with this finding, AVT inhibits GBM proliferation and induces GBM cell apoptosis by inducing CCND1 degradation and arresting the cell cycle at the G1 phase. This finding also shows that targeting the USP10/CCND1 axis could be a potential strategy for the treatment of GBM.
In conclusion, the present study, for the first time, identifies USP10 as a novel deubiquitinase of CCND1 that prevents K48-linked polyubiquitination of CCND1. Because both USP10 and CCND1 are dysregulated in GBM and predict poor prognosis, suppression of the USP10/CCND1 axis with natural products such as AVT might be a novel therapeutic strategy.
References
Khasraw M, Reardon DA, Weller M, Sampson JH. PD-1 inhibitors: do they have a future in the treatment of glioblastoma? Clin Cancer Res. 2020;26:5287–96.
Jackson C, Choi J, Khalafallah AM, Price C, Bettegowda C, Lim M, et al. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J Neurooncol. 2020;148:419–31.
Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl). 2016;94:1313–26.
Jeon S, Kim Y, Jeong YM, Bae JS, Jung CK. CCND1 splice variant as a novel diagnostic and predictive biomarker for thyroid cancer. Cancers (Basel). 2018;10:437. https://doi.org/10.3390/cancers10110437.
Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–8.
Mahzouni P, Taheri F. An Immunohistochemical study of cyclin D1 expression in astrocytic tumors and its correlation with tumor grade. Iran J Pathol. 2019;14:252–7.
Ciznadija D, Liu Y, Pyonteck SM, Holland EC, Koff A. Cyclin D1 and cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 2011;71:6174–83.
Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24.
Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–66.
Shan J, Zhao W, Gu W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell. 2009;36:469–76.
Gennaro VJ, Stanek TJ, Peck AR, Sun Y, Wang F, Qie S, et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci USA. 2018;115:E9298–E9307.
Magiera K, Tomala M, Kubica K, De Cesare V, Trost M, Zieba BJ, et al. Lithocholic acid hydroxyamide destabilizes cyclin D1 and induces G0/G1 arrest by inhibiting deubiquitinase USP2a. Cell Chem Biol. 2017;24:458–470. e418.
Torsvik A, Stieber D, Enger PO, Golebiewska A, Molven A, Svendsen A, et al. U-251 revisited: genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med. 2014;3:812–24.
Le Mercier M, Mathieu V, Haibe-Kains B, Bontempi G, Mijatovic T, Decaestecker C, et al. Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J Neuropathol Exp Neurol. 2008;67:456–69.
Xu Y, Xu M, Tong J, Tang X, Chen J, Chen X, et al. Targeting the Otub1/c-Maf axis for the treatment of multiple myeloma. Blood. 2020. https://doi.org/10.1182/blood.2020005199.
Sewify EM, Afifi OA, Mosad E, Zaki AH, El Gammal SA. Cyclin D1 amplification in multiple myeloma is associated with multidrug resistance expression. Clin Lymphoma Myeloma Leuk. 2014;14:215–22.
Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403.
Ding K, Ji J, Zhang X, Huang B, Chen A, Zhang D, et al. RNA splicing factor USP39 promotes glioma progression by inducing TAZ mRNA maturation. Oncogene. 2019;38:6414–28.
Zhou A, Lin K, Zhang S, Ma L, Xue J, Morris SA, et al. Gli1-induced deubiquitinase USP48 aids glioblastoma tumorigenesis by stabilizing Gli1. EMBO Rep. 2017;18:1318–30.
Wang C, Zheng Z, Deng X, Ma X, Wang S, Liu J, et al. Flexible and powerful strategy for qualitative and quantitative analysis of valepotriates in Valeriana jatamansi Jones using high-performance liquid chromatography with linear ion trap Orbitrap mass spectrometry. J Sep Sci. 2017;40:1906–19.
Sun J, Li T, Zhao Y, Huang L, Sun H, Wu H, et al. USP10 inhibits lung cancer cell growth and invasion by upregulating PTEN. Mol Cell Biochem. 2018;441:1–7.
Takayama KI, Suzuki T, Fujimura T, Takahashi S, Inoue S. Association of USP10 with G3BP2 inhibits p53 signaling and contributes to poor outcome in prostate cancer. Mol Cancer Res. 2018;16:846–56.
Ouyang SW, Liu TT, Liu XS, Zhu FX, Zhu FM, Liu XN, et al. USP10 regulates Musashi-2 stability via deubiquitination and promotes tumour proliferation in colon cancer. FEBS Lett. 2019;593:406–13.
Grunda JM, Nabors LB, Palmer CA, Chhieng DC, Steg A, Mikkelsen T, et al. Increased expression of thymidylate synthetase (TS), ubiquitin specific protease 10 (USP10) and survivin is associated with poor survival in glioblastoma multiforme (GBM). J Neurooncol. 2006;80:261–74.
Barbash O, Egan E, Pontano LL, Kosak J, Diehl JA. Lysine 269 is essential for cyclin D1 ubiquitylation by the SCF(Fbx4/alphaB-crystallin) ligase and subsequent proteasome-dependent degradation. Oncogene. 2009;28:4317–25.
Bello de Carvalho CM, Maurmann N, Luz DI, Fett-Neto AG, Rech SB. Control of development and valepotriate production by auxins in micropropagated Valeriana glechomifolia. Plant Cell Rep. 2004;23:251–5.
Andreatini R, Sartori VA, Seabra ML, Leite JR. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16:650–4.
Jugran AK, Rawat S, Bhatt ID, Rawal RS. Valeriana jatamansi: an herbaceous plant with multiple medicinal uses. Phytother Res. 2019;33:482–503.
Tiedemann RE, Mao X, Shi CX, Zhu YX, Palmer SE, Sebag M, et al. Identification of kinetin riboside as a repressor of CCND1 and CCND2 with preclinical antimyeloma activity. J Clin Investig. 2008;118:1750–64.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (#81970194 and #81770154 to XLM, and #81770215 to BYC), by Guangzhou Municipal Science and Technology Project (#202002030059 to XLM). XLM is a Nanshan Scholar of Guangzhou Medical University.
Author information
Authors and Affiliations
Contributions
XLM, YK, and YYZ designed the study; TS, YJX, SYJ, ZX, and BYC conducted experiments; XLM, TS, YK, YYZ, and GS analyzed data; XLM and TS wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Sun, T., Xu, Yj., Jiang, Sy. et al. Suppression of the USP10/CCND1 axis induces glioblastoma cell apoptosis. Acta Pharmacol Sin 42, 1338–1346 (2021). https://doi.org/10.1038/s41401-020-00551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-00551-x
Keywords
This article is cited by
-
Immune cell infiltration and drug response in glioblastoma multiforme: insights from oxidative stress-related genes
Cancer Cell International (2024)
-
CircNFATC3 promotes the proliferation of gastric cancer through binding to IGF2BP3 and restricting its ubiquitination to enhance CCND1 mRNA stability
Journal of Translational Medicine (2023)
-
USP13 deubiquitinates and stabilizes cyclin D1 to promote gastric cancer cell cycle progression and cell proliferation
Oncogene (2023)
-
Inhibition of USP10 induces myeloma cell apoptosis by promoting cyclin D3 degradation
Acta Pharmacologica Sinica (2023)
-
USP10 deubiquitinates RUNX1 and promotes proneural-to-mesenchymal transition in glioblastoma
Cell Death & Disease (2023)