Abstract
Impulsivity is a multidimensional heritable phenotype that broadly refers to the tendency to act prematurely and is associated with multiple forms of psychopathology, including substance use disorders. We performed genome-wide association studies (GWAS) of eight impulsive personality traits from the Barratt Impulsiveness Scale and the short UPPS-P Impulsive Personality Scale (N = 123,509–133,517 23andMe research participants of European ancestry), and a measure of Drug Experimentation (N = 130,684). Because these GWAS implicated the gene CADM2, we next performed single-SNP phenome-wide studies (PheWAS) of several of the implicated variants in CADM2 in a multi-ancestral 23andMe cohort (N = 3,229,317, European; N = 579,623, Latin American; N = 199,663, African American). Finally, we produced Cadm2 mutant mice and used them to perform a Mouse-PheWAS (“MouseWAS”) by testing them with a battery of relevant behavioral tasks. In humans, impulsive personality traits showed modest chip-heritability (~6–11%), and moderate genetic correlations (rg = 0.20–0.50) with other personality traits, and various psychiatric and medical traits. We identified significant associations proximal to genes such as TCF4 and PTPRF, and also identified nominal associations proximal to DRD2 and CRHR1. PheWAS for CADM2 variants identified associations with 378 traits in European participants, and 47 traits in Latin American participants, replicating associations with risky behaviors, cognition and BMI, and revealing novel associations including allergies, anxiety, irritable bowel syndrome, and migraine. Our MouseWAS recapitulated some of the associations found in humans, including impulsivity, cognition, and BMI. Our results further delineate the role of CADM2 in impulsivity and numerous other psychiatric and somatic traits across ancestries and species.
Similar content being viewed by others
Introduction
Impulsivity is a multifaceted psychological construct that has been broadly defined as thoughts or actions that are “poorly conceived, prematurely expressed, unduly risky or inappropriate to the situation, and that often result in undesirable consequences” [1]. Impulsivity has been repeatedly associated with numerous psychiatric diseases, including ADHD and substance use disorders [2, 3]. We previously performed genome-wide association studies (GWAS) of impulsive personality traits (n = 21,806–22,861) using two of the most widely used impulsivity questionnaires, the Barratt Impulsiveness Scale (BIS-11; 3 traits) and the Impulsive Personality Scale (UPPS-P; 5 traits), as well as a measure of Drug Experimentation [4]. These traits were partially genetically correlated, suggesting that each impulsivity domain is governed by overlapping but distinct biological mechanisms [4, 5]. Our work also identified significant genetic correlations between impulsivity and numerous psychiatric and substance use traits, in line with the NIMH Research Domain Criteria (RDoC), proposing impulsivity as a transdiagnostic endophenotype for psychopathology [6].
The cell adhesion molecule 2 (CADM2) gene, which was the most robustly implicated gene in our prior GWAS of impulsivity [4], has also been extensively implicated in other risky and substance use behaviors [7]. CADM2 mediates synaptic plasticity and is enriched in the frontal cortex and striatum, which are regions that regulate reward and inhibitory processes. We and others have implicated this gene in traits that may underlie disinhibition in humans, supporting the observed genetic correlations between impulsivity and personality [8], educational attainment [9], cognition [10], risk-taking [11], substance use [4, 10, 12,13,14,15], externalizing psychopathology [16], neurodevelopmental disorders [17, 18], physical activity [19], reproductive health [20, 21], metabolic traits [22], and BMI [23], among others (see GWAS Catalog www.ebi.ac.uk/gwas/). Cadm2 knockout mice have previously been assessed for body weight and energy homeostasis [24] but have never been behaviorally characterized for measures of impulsivity or related behaviors.
Here, we took three approaches to elucidate genetic factors related to impulsivity. First, we collaborated with 23andMe, Inc., to extend upon our earlier GWAS of impulsivity [4] by increasing our sample size approximately 6-fold (n = 123,509–133,517). Second, we performed single-SNP phenome-wide studies (PheWAS) of the 5 single nucleotide polymorphisms (SNPs) in and around CADM2 that have been most strongly implicated by the current and prior GWAS. PheWAS were conducted in three ancestral groups (N = 3,229,317, European; N = 579,623, Latin American; N = 199,663, African American) from the 23andMe research cohort, examining close to 1300 traits, most with no published GWAS. Finally, we performed a mouse-PheWAS (“MouseWAS”) by creating and phenotyping mice harboring a Cadm2 mutant allele in a broad battery of behavioral tasks that included analogous human measures of risk-taking and impulsivity, substance use, cognition and BMI.
Materials and methods
Human studies
GWAS cohort and phenotypes
We analyzed data from a cohort of up to 133,517 male and female research participants of European ancestry, a subset of which were analyzed in our prior publications [4, 13, 25, 26]. All participants were drawn from the research participant base of 23andMe, Inc., a direct-to-consumer genetics company, and were not compensated for their participation. Participants provided informed consent and participated in the research online, under a protocol approved by the external AAHRPP-accredited IRB, Ethical & Independent Review Services (www.eandireview.com). During 4 months in 2015 and 14 months from 2018–2020, participants responded to a survey that included up to 139 questions pertaining to aspects of impulsivity and substance use and misuse. To measure impulsive personality, we used five subscales from the UPPS-P ([27, 28]; a 20-item that measures (lack of) Premeditation, (lack of) Perseverance, Positive Urgency, Negative Urgency, and Sensation Seeking; Table S1). We also administered the BIS-11 ([29]; a 30-item questionnaire that measures Attentional, Motor, and Nonplanning impulsiveness; Table S1). Lastly, we measured Drug Experimentation, defined as the number of substances an individual has used (adapted from the PhenX toolkit [30]; Table S1). We scored UPPS-P, BIS-11 and Drug Experimentation as previously described [4]. We used quantile normalization, since some scores were not normally distributed (Figs. S1–3). Only individuals identified as being of European ancestry based on empirical genotype data [31] were included in this study. Basic demographic information about this sample is presented in Table S2. We used Pearson correlation coefficients (r) to measure the phenotypic relationships between impulsivity subscales and demographics.
Genome-wide association and secondary analyses
DNA extraction and genotyping were performed on saliva samples by CLIA-certified and CAP-accredited clinical laboratories of Laboratory Corporation of America. Quality control, imputation, and genome-wide analyses were performed by 23andMe (Table S3; [32, 33]). 23andMe’s analysis pipeline performs linear regression assuming an additive model for allelic effects (Supplementary Material). Covariates included age (inverse-normal transformed), sex, the top five principal genotype components, and indicator variables for genotyping platforms. p-values were corrected for genomic control. We examined genotype*sex interactions for suggestive loci. In addition, for the top loci, we examined African American and Latin American 23andMe research participants who had responded to the same survey.
We used the FUMA web-based platform (version 1.3.6a) and MAGMA v1.08 [34, 35] to explore the functional consequences of the GWAS loci and to conduct gene-based analyses.
We used LDSC [36] to calculate genetic correlations (rg) between UPPS-P, BIS and Drug Experimentation, and 96 selected traits informed by prior literature.
Phenome-wide association scan (PheWAS) in 23andMe
We performed single-SNP PheWAS for 5 CADM2 SNPs (rs993137, rs62263923, rs11708632, rs818219, rs6803322) using up to 1291 well-curated self-reported phenotypes from a separate cohort of 23andMe research participants of European (N ≤ 3,229,317), Latin American (N ≤ 579,623) and African American (N ≤ 199,663) ancestries. We excluded traits with <1000 responses, based on a prior simulation study for PheWAS power analysis [37]. Ancestry was determined by analyzing local ancestry ([31] Supplementary Material). The variants were selected based on our GWAS results and previous literature (Table S4, and Supplementary Material). Genotyped and imputed variant statistics for the PheWAS are shown in Table S5.
An overview of the data collection process has been previously described [38]. All regression analyses were performed using R version 3.2.2. We assumed additive allelic effects and included covariates for age (as determined by participant date of birth), sex, and the top five ancestry-specific principal components. We used a 5% FDR correction for multiple testing.
MouseWAS
Subjects, behavioral characterization, and statistical analyses
Our Cadm2 mutant mice were produced at the University of California San Diego, Moores Cancer Center, Transgenic Mouse Core. We used the JM8.N4 cryosperm line (CSD70565 KOMP), which carries a floxed null allele in the Cadm2 gene (Fig. S30), on a C57BL/6 N background. We crossed the floxed null allele line with a constitutive CRE driver line (Stock# 014094; The Jackson Laboratory), yielding a global constitutive null allele. We used a heterozygous x heterozygous (HET) breeding scheme, which produced homozygous (HOM) mutant Cadm2 mice and their HET and wildtype (WT) littermates. Mice were genotyped using allele-specific polymerase chain reaction on ear notch tissue followed by gel electrophoresis [39]. CADM2 protein expression levels were quantified by western blotting (Fig. S31).
Five separate cohorts of male and female mice were used for these studies. See Supplementary Material for a more detailed description of the tasks and analyses of main variables. Procedures were approved by the University of California San Diego Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care. Procedures from cohort 2 were conducted in accordance with the Canadian Council on Animal Care and were approved by the University of Guelph Institutional Animal Care and Use Committee.
Results
Genome-wide association analyses and secondary analyses
Self-reported impulsivity and drug experimentation scores are shown in Table S6. We found that ~6–11% of the phenotypic variation of these traits can be explained by common variants (Table S7). We identified 21 genome-wide significant associations (p < 5.0E-08) for UPPS-P (5 traits), BIS (3 traits), and Drug Experimentation (Figs. 1; S4–21; Table S8). Although we tested 9 traits, in keeping with the standards of the field, we did not adjust the significance threshold. We also detected several nominal associations (p < 1.0E-06, Table S8); we discuss some of them in the Supplementary Material.
CADM2 was consistent across 3/8 impulsivity facets [Sensation Seeking (UPPS-P), Motor and Nonplanning impulsivity (BIS-11)] at a genome-wide association level, and with 3 more impulsivity facets [Attentional (BIS-11), Negative Urgency and Premeditation (UPPS-P]), at a gene-based level (Table S8). CADM2 was also associated with risky behavior, such as Drug Experimentation.
GWAS of UPPS-P
Premeditation
We detected one significant hit (rs2958162, p = 2.50E-10), located on chromosome 18 in the TCF4 gene, which encodes a helix-loop-helix transcription factor and is widely expressed throughout the body and during development. Polymorphisms in TCF4 have been associated with risk-taking and adventurousness [15], alcohol consumption [40], schizophrenia [41], depression [42, 43], and neuroticism [44, 45] (Table S9); TCF4 is also a non-GWAS candidate gene for other psychiatric and neurological conditions [46].
Perseverance
We detected one significant association (rs5943997, p = 1.50E-8) in the POLA1 gene on the X chromosome. POLA1 has been related to blood traits [46] and neurodevelopmental disorders [47], but its association with impulsivity is novel.
Positive urgency
We identified one significant hit (rs143987963, p = 4.30E-08) on chromosome 12, near the genes MDM1 and RAP1B; however, inspection of the locus zoom plot (Fig. S9) does not support a robust association.
Negative urgency
We detected three significant hits: rs4840542 (p = 1.60E-09), on chromosome 8, in the XKR6 gene; rs5008475 (p = 4.90E-09), on chromosome 5, near TMEM161B and MEF2C; and rs7829975, on chromosome 8, near SGK223 and CLDN23 (p = 5.00E-09). Variants in strong LD with rs4840542 and rs7829975 are highly pleiotropic, and have been previously associated with several traits (Table S9), including body mass index (BMI) [48, 49], neuroticism [50, 51], depression [52], blood pressure, and alcohol consumption [53]. XKR6 was also implicated in a recent GWAS of externalizing [16], and a GWAS of anxiety and depression [52].
Sensation seeking
We detected 5 significant associations. First, we again observed a previously reported [4] association with a SNP near CADM2 (rs11288859, p = 2.10E-09) on chromosome 3. We also detected an association with a SNP in TCF4 (rs2958178, p = 3.80E-12). We identified a significant hit in CACNA2D1 (rs38547, p = 2.10E-08) on chromosome 18. CACNA2D1 has been previously associated with feeling nervous [50], and levels of sex hormone-binding globulin [54]. Furthermore, we found a significant association (rs1605379, p = 3.80E-08) on chromosome 16, near CYLD and SALL1. SNPs in strong LD with rs1605379 have been previously identified for risk-taking, adventurousness, and smoking initiation (Table S9). Lastly, we found a significant association (rs12600879, p = 4.10E-08) on chromosome 17, near TBX21 and OSBPL7. Variants in strong LD with rs12600879 have been associated with BMI [55], but the finding in relation to impulsivity is novel.
GWAS of BIS-11
Attentional
We identified one significant association (rs10196237, p = 1.10E-08) on chromosome 2, near the genes SPHKAP and PID1. SPHKAP has been previously associated with educational attainment [9], but the association with impulsivity is novel.
Motor
We detected one significant association near CADM2 (rs35614735, p = 3.20E-11). We also identified an association (rs111502401, p = 2.00E-08), on chromosome 19, near the genes ZNF229 and ZNF180; however, inspection of the regional association is not supportive of a strong association (Fig. S17).
Nonplanning
We detected 2 variants: rs35614735 (p = 4.70E-12) near CADM2, which was the same SNP identified for Motor impulsivity; and rs6872863 (p = 1.20E-08) in the gene ELOVL7 on chromosome 5. Variants in strong LD with rs6872863 have been reported for a variety of traits including educational attainment, mathematical ability [9], household income [56], and brain morphology, such as cortical surface area [57] (Table S9). However, there is extensive LD in this region, making the association difficult to interpret.
GWAS of drug experimentation
We previously reported [4] a suggestive association (rs2163971, p = 3.00E-07) near the CADM2 gene. In the present study, we identified a nearby SNP that was genome-wide significant (rs35614735, p = 2.80E-15). We also report 4 novel hits (rs951740, p = 9.70E-10, PTPRF on chromosome 1; rs12713405, p = 9.70E-09, BLC11A on chromosome 2; rs67660520, p = 7.60E-09, CADPS2 on chromosome 7; rs7128648, p = 2.50E-09, NCAM1 on chromosome 11). Intriguingly, PTPRF has been recently associated with problematic prescription opioid use [25] and opioid use disorder [58], as well as smoking initiation/cessation [59], cognition [60], and educational attainment [9] (Table S9). Variants in strong LD with rs67660520 have been associated with ADHD [61], smoking initiation [59], number of sexual partners [15] and BMI [49] (Table S9). NCAM1 variants have been previously associated with alcohol, cannabis and smoking behaviors [59, 62], mathematical ability [9], and anxiety and depression [52], among other traits.
Gene-based analyses
Similar to the GWAS results, gene-based analyses using MAGMA identified an association (Bonferroni p < 2.53E-06; Table S10) between CADM2 and 6 of the 9 traits examined in this paper: Premeditation, Sensation Seeking (UPPS-P); Attentional, Motor and Nonplanning (BIS-11); and Drug Experimentation. TCF4, which was significantly associated with Premeditation and Sensation Seeking in the GWAS, was significantly associated with these traits in the gene-based analysis. MAPT, which has been previously associated with many traits including multiple alcohol-related behaviors [13], was implicated in Negative Urgency. Lastly, KDM4A, which was recently related to problematic opioid use and interacts with selective serotonin reuptake inhibitors and dopaminergic agents [25], was significantly associated with Drug Experimentation.
Phenotypic and genetic correlations
A phenotypic and genetic correlation matrix of all 9 traits is shown in Fig. S22 and Tables S11–12. Consistent with the literature and our prior work [4, 5, 63, 64], both phenotypic and genetic inter-correlations among the UPPS-P and BIS subscales were high and positive, with the exception of Sensation Seeking and Perseverance, suggesting that these traits may represent relatively different constructs [5, 13, 63]. Drug experimentation was positively and significantly associated with all impulsive personality traits.
All impulsivity traits were phenotypically associated (r = −0.34–0.11) with demographic variables (Table S12), impulsivity scores being greater in male and younger research participants, compared to female and older participants; and in participants with higher BMI, lower household income, and fewer years of education, as we previously reported [13].
Figure 2 shows a genetic correlation matrix of BIS, UPPS-P, Drug Experimentation and several other phenotypes (full results in Table S13). As anticipated, we found positive moderate to high genetic correlations (rg = 0.25–0.79) between virtually all UPPS-P (except Perseverance and Sensation Seeking) and BIS subscales, and Drug Experimentation, and substance use disorders Table S13).
Genetic correlations (rg) between UPPS-P, BIS, and Drug Experimentation, and other substance use, psychiatric, personality, cognitive, metabolic, health, pain, longevity and sleep traits (see Table S9 for full results). All values survive 5% FDR correction.
We also observed moderate to strong associations between all impulsive subscales (except UPPS-P Perseverance) and other personality traits, such as risk-taking (rg = 0.15–0.65), neuroticism (rg = −0.23–0.84), and loneliness (rg = 0.17–0.54), particularly for Positive and Negative Urgency. Extraversion was positively associated with Sensation Seeking (rg = 0.34). Externalizing psychopathology, which represents disorders and behaviors characterized by deficits in inhibition, was strongly associated with all impulsivity facets (rg = 0.28–0.92), except Perseverance.
We also identified positive associations with an array of psychiatric phenotypes, including ADHD (rg = 0.20–0.47), depression (rg = −0.13–0.47) and anxiety (rg = −0.38–0.61) disorders, and cross-disorder (rg = 0.12–0.44). The associations were again primarily significant for all except Perseverance and Sensation Seeking. Other disorders showed weaker associations (e.g., schizophrenia, rg = −0.09–0.15) or were only significantly associated with one impulsivity facet [e.g., anorexia nervosa (Perseverance, rg = −0.16); bipolar disorder (Motor, rg = 0.22)].
Most impulsivity subscales were genetically correlated with lower socioeconomic variables [e.g., educational attainment (rg = −0.49 to −0.16), income (rg = −0.38 to −0.16), Townsend index (rg = 0.18–0.58)].
Metabolic and medical phenotypes, such as BMI (rg = 0.18–0.28), chronic pain (rg = 0.22–0.46), insomnia (rg = 0.20–0.42), and coronary artery disease (rg = 0.18–0.30), were genetically correlated with all impulsive subscales (except Perseverance and Sensation Seeking). We also noted negative genetic associations with parental longevity (rg = −0.17 to −0.32).
PheWAS
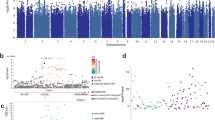
To explore the impact of specific variants in and around CADM2, we performed single-SNP PheWAS using 5 of the most implicated SNPs, independently, against 1291 traits (Fig. 3). The list of PheWAS association results using the 23andMe cohort after 5% FDR correction is available in Table S14 (summary), S15 (Europeans), S16 (Latin American) and S17 (African Americans).
FDR-significant associations from CADM2 single-SNP PheWAS in individuals of European ancestry (A) and Latin American ancestry (B). Results for the SNP with the highest number of significant PheWAS associations (rs993137) are presented; results for additional SNPs are included in Supplementary Tables 15–17. No FDR-significant findings were detected in individuals of African American ancestry. The size of the dots represents the magnitude of the effect size for each trait. The effect sizes ranged from −0.14 to 0.13 in the European cohort and from −0.08 to 0.16 in the Latin American cohort. Dotted line denotes Bonferroni significance (p < 3.79E-05).
In European cohorts, CADM2 variants had been previously identified to be significantly associated with numerous traits (Table S18). Most SNPs were highly correlated (R2 > 0.1) and tagged similar traits (Fig. S23), but the overlap was incomplete (Fig. S24 and Table S19). rs993137, located at 85,449,885 bp on chromosome 3, showed the highest number of associations (378), which we describe below.
We replicated all previously known associations in 23andMe participants of European ancestry, identifying signals across all categories tested (Table S15). These included negative associations with risky behavior (e.g., lower risk for adventurousness [β = −0.05, p = 1.33E-08], risk-taking tendencies [β = −0.02, p = 1.13E-07]) and substance use behaviors (e.g., lower risk for alcohol consumption [β = −0.03, p = 2.05E-09] and tobacco initiation (β = −0.02, p = 3.66E-12; but see packs per day, β = 0.01, p = 1.05E-03), as well as negative associations with psychiatric disorders characterized by deficits in impulsivity, such as lower risk for ADHD (β = −0.05, p = 2.17E-41). Furthermore, we found positive associations with educational outcomes (e.g., higher educational attainment (β = 0.03, p = 1.67E-12). Novel findings included positive associations with allergies (β = 0.04, p = 4.51E-03), anxiety (e.g., panic [β = 0.02, p = 6.82E-08]), and medical conditions (e.g., IBS [β = 0.02, p = 8.89E-07]), anemia (β = 0.01, p = 8.30E-74), hepatitis C (β = −0.06, p = 8.36E-10). Intriguingly, we also detected positive associations with pain phenotypes (β = 0.02, p = 8.37E-12) and a need for a higher dose of pain medication (β = 0.01, p = 1.02E-06).
For the overlapping phenotypes, UK Biobank PheWAS results [65] largely supported the 23andMe PheWAS findings (except for smoking behaviors). For example, we identified associations with dietary traits (e.g., daily fruit and vegetable intake (β = −0.01, p = 4.23E-11), pastry frequency (β = 0.01, p = 7.36E-06), sleep quality (β = −0.01, p = 2.53E-03), and number of pregnancies (β = −0.01, p = 7.69E-04), among others (Table S15, [12]).
In the PheWAS of the Latin American cohort, 47 traits were significantly associated with CADM2 variants (Table S16). The highest number of associations were again observed for rs993137 [66], which are described below. Similarly, although some of the SNPs were correlated (R2 > 0.1; Fig. S24), the overlap was incomplete (Fig. S26; Table S20). The pattern of associations was consistent with those described in the European cohort. The strongest associations were with risky behaviors, such as adventurousness (β = −0.04, p = 1.76E-17), risk-taking (β = −0.02, p = 5.90E-07), alcohol consumption (β = −0.03, p = 1.41E-12), and disorders characterized by high levels of impulsivity, such as ADHD (β = −0.04, p = 4.74E-10). The novel findings were, again, with multiple forms of allergies (e.g., seasonal allergies, β = 0.03, p = 3.0E-04), migraine (β = 0.04, p = 1.56E-04), sleep behaviors (e.g., sleep apnea, β = −0.03, p = 6.76E-04), among others.
All findings that were in common between the European and Latin American cohorts showed the same direction of effect and similar effect sizes. We did not identify FDR-significant associations in the African American cohort (Table S17). The effect sizes were generally extremely small (Figs. S27–28), as is expected for a single gene and complex traits.
MouseWAS
Figure 4 summarizes the MouseWAS results across the five cohorts tested. Full statistics and additional secondary measures are described in the Supplementary Material and Table S20.
MouseWAS examined the complete and partial loss of Cadm2 function and behavioral consequences in the Probability Breakpoint Ratio Task (PBRT, A), Probabilistic Reversal Learning Task (PRLT, B), Iowa gambling task (IGT, C–D), Behavioral Pattern Monitor task (BPM, E–F), Prepulse Inhibition (PPI, G–H), 5-Choice Serial Reaction Time Task (5CSRTT) performance (I–M), Locomotor (LCM) activity (N), Elevated Plus Maze (EPM, O), Light-Dark Box (LDB, P), and Drinking in the Dark (DID, Q); longitudinal body weight changes (R), and dendrite morphology (DSM) in the nucleus accumbens (S). Western blot (WB) analysis (T) of Cadm2 protein in whole brain, frontal cortex and striatum. WT wildtype, HET heterozygote, HOM homozygote. Sample size by cohort: cohort 1 (WT = 25, HET = 30, HOM = 3), cohort 2 (WT = 13; HET = 14, HOM = 12), cohort 3 (WT = 22; HET = 44, HOM = 12), cohort 4 (WT = 29, HT = 54, HOM = 17), cohort 5 (WT = 3, HET = 3, HOM = 3). In cohort 1, the sample size of the HOM mice deviated from the expected Mendelian frequency for unknown reasons; these animals were excluded from the analyses. Males are represented in circles, females in triangles. *p < 0.05, **p < 0.01, ***p < 0.001.
Cohort 1 - Motivation, inhibition, and risk-taking behavior
No differences in motivation were found between WT and HET mice during the Progressive Breakpoint task [F(1,51) = 0.003, p = 9.57E-01; Fig. 4A]. However, we noted significant genotype differences in behavioral flexibility in the Probabilistic Reversal Learning (PRL) task, as indexed by the number of trials to first reversal [F(1,42) = 4.27, p = 4.50E-02; Fig. 4B], and risky behavior in the mouse Iowa Gambling Task (IGT; F(1,51) = 4.70, p = 3.50E-02; Fig. 4C], HET mice requiring fewer trials to reach criterion and choosing risky options less frequently than WT mice (p < 0.05), respectively. The number of premature responses, on the contrary, were higher in HET mice [F(1,51) = 5.78, p = 2.00E-02] compared to WT mice (p < 0.05; Fig. 4D). In the Behavioral pattern monitor (BPM), HET mice exhibited greater exploratory behavior, as shown by an increase in hole-pokes [F(1,53) = 4.88, p = 3.20E-02; Fig. 4E], compared to WT mice (p < 0.05), but general levels of activity, such as distance traveled (F(1,53) = 0.42, p = 5.21E-01; Fig. 4F), were similar across the genotypes. Lastly, although the startle response was equal across the groups (Fig. 4G), prepulse inhibition (PPI) was larger in HET mice compared to WT mice (p < 0.05; Fig. 4H), particularly at ISI 25 and 100 in HET mice [F(1,53) = 8.23, p = 6.00E-03, F(1,53) = 4.50, p = 3.90E-02, respectively].
Cohort 2 - Motoric impulsivity
The main outcome tested in cohort 2 were premature responses via the 5-choice serial reaction time task (5CSRTT; Fig. 4J–M). Premature responses were lower in HOM (p < 0.001) and WT (p < 0.02) mice compared to HET mice under standard conditions (F(2,36) = 8.74, p = 8.06E-04; Fig. 4J), and compared to both HET (p < 0.001) and WT (p < 0.01) mice during a long ITI session (H(2) = 16.10, p = 3.19E-04; Fig. 4L). HOM mice were faster at learning the 5CSRTT, requiring fewer days for adequate baseline performance (F(2,36) = 7.42, p = 2.00E-03; Fig. 4I), compared to WT mice (p < 0.01).
Cohort 3 - General locomotion, anxiety-like behavior, and ethanol consumption
We found a significant effect of genotype on the distance traveled in the Open Field [OF; F(2,70) = 7.525, p = 1.00E-03; Fig. 4N], with HOM mice showing higher levels of locomotor activity than WT mice (p = 1.40E-02). No differences in anxiety-like behavior were detected across WT, HET or HOM mice in the Elevated Plus Maze (EPM) or Light-Dark Box (LDB) tests (Fig. 4O–P; Table S20). The total amount of ethanol consumed during the drinking-in-the-dark (DID) paradigm did not differ between the groups ([F(2,78) = 1.084, p = 3.44E-01]; Fig. 4Q).
Cohort 4 - Body weight
Relative to WT mice, there was a significant reduction in body weight in HOM mice from week 21 onwards (β = −3.74 ± 1.27, p = 4.00E-03; Fig. 4R).
Cohort 5 - Dendrite morphology
Quantitative analyses of MSN in the NAc revealed no difference in dendritic spine density across the groups (Fig. 5S).
Discussion
In this study, we performed the largest GWAS of impulsive personality traits to date, we conducted the first multi-ancestral PheWAS exploring the role of CADM2 on a diverse array of traits, and we performed a corresponding MouseWAS using Cadm2 mutant mice to assess its role in impulsivity and other relevant behaviors. We extended on prior findings [4, 5] showing that the genetic architectures of impulsivity facets only partially overlap, providing further support to the idea of impulsivity being a multifaceted construct even at the genetic level. We identified positive genetic correlations across multiple domains, particularly substance use disorders, confirming that NIMH RDoC transdiagnostic domains [6], or endophenotypes, such as impulsive personality traits, can be used to dissect the genetic basis of psychiatric illness and normal functioning. RDoC or transdiagnostic traits are beneficial because they enable translational research and provide a more granular biological understanding of psychiatric disorders. Using mouse and human correlates, we provided further evidence that CADM2 is a robust candidate gene for impulsivity and an important modulator of numerous other psychiatric and somatic traits.
We increased the sample size of our prior GWAS of impulsivity by almost 6-fold and identified 21 genome-wide significant loci implicated in impulsive personality and Drug Experimentation. For instance, SNPs located in the gene TCF4 were implicated in 3 subscales; this gene is also highly pleiotropic for other psychiatric conditions. Furthermore, we identified associations with NCAM1, which, intriguingly, is a critical member of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster [67] and variants correlated with NCAM1 in that cluster have been associated with differences in D2 receptor density [68]. We also detected associations near XKR6 and AFF3, which have been recently implicated in externalizing psychopathology [16], and PTPRF and KDM4A, recently implicated in problematic opioid use [25] and opioid use disorder [58]. Although in this report we focused on CADM2, functional studies of those genes are also warranted. Furthermore, we found nominal evidence for candidate gene studies implicating monoamine neurotransmitters in impulsivity and Drug Experimentation (DRD2, HTR3B). High impulsivity depends on a neural network that includes the ventral striatum (subsuming the NAc) with top-down control from prefrontal cortical regions, and is modulated by monoamine neurotransmitters including dopamine and serotonin [69]; this is the first GWAS to implicate genes modulating these systems as robust candidate genes for impulsivity.
Recent studies have implicated the CADM2 gene in impulsivity and traits associated with reward sensitivity and multiple domains of human health. We confirmed numerous previously reported associations and extended our findings of variants related to CADM2. CADM2 was significantly associated with 4 out of the 9 traits that we measured in GWAS and 6 out of the 9 traits that we measured in gene-based analyses. In the PheWAS, CADM2 variants were associated with decreased risk for externalizing psychopathology, but also increased risk for internalizing psychopathology (anxiety, depression, OCD). We also observed novel associations with migraines and various allergies. Using a similar approach with UK Biobank data, previous studies have found that this enrichment of associations is higher than expected [12] compared to other genes. These results provide evidence that CADM2 variants are associated with broad health outcomes, but whether this gene affects human health via disruptions in inhibitory control or reward systems, or whether it acts via multiple pathways [70], is still not fully understood.
A relatively unique feature of our study is that, to follow up on the CADM2 loci implicated in human studies, we generated a Cadm2 mutant mouse line and used it to perform a PheWAS-like study in mice, which we have termed a MouseWAS to emphasize its conceptual similarity to human PheWAS studies. These functional experiments provided information about the causality and directionality of effects in its reported associations. We found evidence that loss of Cadm2 resulted in less risky behavior and improved information processing, extending on prior work in humans [4, 10, 16, 68, 71].
Cadm2 expression may uniquely contribute to the different domains of impulsivity. The IGT assays preference for high risk, high reward (disadvantageous) choices vs low risk, low reward (advantageous) choices [72]. HET mice exhibited a greater preference for selecting the safe option vs their WT littermates. This finding can be contrasted with the elevated premature responses in the 5CSRTT seen in HET vs WT mice, reflective of motoric impulsivity. However, premature responses have also been linked to temporal discrimination, wherein mice and humans overestimating the passage of time exhibit higher premature responses [73, 74]. The preference for less risky options of HET mice in the IGT could reflect their misjudgment of time – resulting in higher premature responses – and thus avoidance of higher temporal punishment in the IGT.
We also observed genotype differences in performance that could be indicative of Cadm2 function in information processing. HET mice exhibiting better PPI at the shortest temporal window (25 ms) supports the premise that these mice have faster processing speeds. HET mice also showed small increases in hole-poking in the BPM test, which is thought to reflect exploration of the environment and information gathering. Finally, we observed that HOM mice acquired 5CSRTT faster than WT littermates. Taken together, these results suggest that Cadm2 reduction may improve some facets of information processing.
Findings from the 5CSRTT provide evidence that Cadm2 deletion improves some information processing and impulsivity outcomes, while being detrimental to others. HET mice were the most likely to commit 5CSRTT premature responses, although HOM mice were surprisingly the least likely to make premature responses. Interestingly, although not significant, there was a consistent elevation in the number of premature responses committed by the HOM mice as the stimulus duration was reduced. This could suggest that HOM mice, like HET mice, may show motoric impulsivity deficits when performing tasks that require greater attentional demand. Compared with WT, HOM mice also showed impaired accuracy performance under RSD conditions, in line with our human findings of CADM2 association with BIS Attentional, and cognitive function by others [10]. The heterogeneity of performance outcomes in the HOM mice further supports a unique but overlapping contribution of genetics across impulsivity domains.
In this paper, we translated measures from human to mice. These studies begin the process of understanding the biological basis of associations identified by GWAS. The methods for measuring impulsivity in humans and mice are fundamentally different. Despite these differences, our MouseWAS identified several measures of impulsivity that were influenced by Cadm2, consistent with our observations in humans. Furthermore, CADM2 has been shown to be implicated in BMI in humans [24, 75] and energy homeostasis in mice [24]; extending on this, we found novel evidence of body weight reductions in adult mutant mice. Interestingly, Cadm2 did not have more general effects on mouse behavior; for instance, we did not observe deficits in anxiety-like behavior or general motivation, as some of the human PheWAS findings revealed. A few other measures were also inconsistent across species, particularly measures of alcohol consumption, where CADM2 showed a role in humans [7, 13, 76, 77] but not mice. Lastly, some measures identified by our human PheWAS (e.g., allergies and other medical conditions) were not examined in our MouseWAS. This approach highlights the challenges of using mouse models to further investigate the role of specific genes in behavioral traits.
CADM2 encodes the immunoglobulin adhesion protein SynCAM 2, which is part of the family of synaptic adhesion molecules known as SynCAMs. Studies have shown the influence of SynCAMs on synaptogenesis [78,79,80,81,82], axon guidance [83], and neuron myelination [84,85,86], processes that have direct effects on the pathology of neurodevelopmental diseases [56]. CADM2 is strongly expressed in the striatum and frontal cortex, which are core regions that regulate impulsivity [69]. We did not observe changes in spine density in the Nac, which suggests that Cadm2 may not have a role as a postsynaptic organizer of spines in this region, or may have redundant functions that are compensated in the mutant mice by other molecules. Based on in-silico analyses in humans, CADM2 expression seems to be greater at earlier stages of development (Fig. S29); whether Cadm2 may affect earlier stages of development (prenatal and early postnatal) that are compensated in adulthood has not been investigated in this study.
Several limitations of this study are worth noting. The discovery GWAS only includes male and female participants of European ancestry. While we provided exploratory analyses of top variants in other ancestries and broken down by sex (Supplementary Table 22), larger sample sizes would be needed to perform GWAS separately in males and females. Our results are also biased by potential ascertainment and characteristics of the sample; the 23andMe participant population is more educated and has higher socioeconomic status and lower levels of drug use and impulsivity than the general US population [86]. Replication in additional cohorts with different characteristics is warranted. Moreover, although the traits we studied are extracted via well-established questionnaires, they are self-reported measures, which are different from behavioral phenotypes [87, 88]. Another issue is that, although we tested multiple variants in the CADM2 loci, further conditional analyses are required to determine if this signal and previously reported associations implicating CADM2 loci, including a large non-coding rare deletion in the first intron of CADM2 [70], may tag the same underlying genetic effect. We are also unaware of the sequence of events, and whether there is true pleiotropy or mediation effects has not been examined. The analyses were well-powered for moderate and large effect sizes. Still, for unclear reasons, despite similar minor allele frequencies and imputation quality of the SNPs we tested across all ancestries, we identified no significant associations in the African American cohort. Finally, although our mouse studies detected some discordant cross-species effects of Cadm2 on behavior, background strain effects [89] or subtle allelic variations (vs whole KO) may explain those differences. While some results are suggestive of additive effects, we were unable to evaluate different genetic models due to lack of sufficient sample sizes for HOM mice. Future multivariate analyses examining paths of commonality and specificity across impulsivity facets may provide further insights not herein examined.
In conclusion, we show that impulsivity facets are extremely polygenic, but of very high transdiagnostic significance. Genetic studies using research participants not ascertained for neuropsychiatric disorders may represent an efficient and cost-effective strategy for elucidating the genetic basis and etiology of genetically complex psychiatric diseases. Using homologous measures of impulsivity in mice and humans across three ancestral backgrounds, we provide evidence of the overarching role of CADM2 on impulsivity, and a much broader impact on human health.
Data availability
We provide summary statistics for the top 10,000 SNPs (Tables S23–31). Full GWAS summary statistics will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#dataset-access/ for more information and to apply to access the data.
Code availability
All software used to generate results has been previously published, and corresponding citations are provided in the Methods.
References
Daruna JH, Barnes PA. A neurodevelopmental view of impulsivity. Impuls. Client Theory Res. Treat., Washington, D.C.: American Psychological Association; 1993. p. 23.
Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcohol Clin Exp Res. 2013;37:1441–50.
Jackson JNS, MacKillop J. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:316–25.
Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, MacKillop J, et al. Genome-wide association studies of impulsive personality traits (BIS-11 and UPPSP) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J Neurosci. 2019;39:2562–72.
Gustavson DE, Friedman NP, Fontanillas P, Elson SL. The latent genetic structure of impulsivity and its relation to internalizing psychopathology. Psychol Sci. 2020;31:1025–35.
Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7.
Arends RM, Pasman JA, Verweij KJH, Derks EM, Gordon SD, Hickie I, et al. Associations between the CADM2 gene, substance use, risky sexual behavior, and self-control: a phenome-wide association study. Addict Biol. 2021;26:e13015.
Boutwell B, Hinds D, Tielbeek J, Ong KK, Day FR, Perry JRB, et al. Replication and characterization of CADM2 and MSRA genes on human behavior. Heliyon. 2017;3:e00349.
Lee JJ, Wedow R, Okbay A, 23andMe Research Team, COGENT (Cognitive Genomics Consortium), Social Science Genetic Association Consortium et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21.
Ibrahim-Verbaas CA, Bressler J, Debette S, Schuur M, Smith AV, et al. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol Psychiatry. 2016;21:189–97.
Strawbridge RJ, Ward J, Cullen B, Tunbridge EM, Hartz S, Bierut L, et al. Genome-wide analysis of self-reported risk-taking behaviour and cross-disorder genetic correlations in the UK Biobank cohort. Transl Psychiatry. 2018;8:39.
Pasman AJ, Chen Z, Vink JM, Van Den Oever MC, Pattij T, Vries TJD, et al. The CADM2 gene and behavior: a phenome-wide scan in UK-Biobank. Behav Genet. 2022;52:306–14.
Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, the 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium, Adams MJ, et al. Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. Am J Psychiatry. 2019;176:107–18.
Pasman JA, Verweij KJH, Gerring Z, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat Neurosci. 2018;21:1161–70.
Linnér KR, Biroli P, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51:245–57.
Linnér RK, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021;24:27.
Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R, et al. A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum Genet. 2012;131:565–79.
Demontis D, Walters RK, Martin J, ADHD Working Group of the Psychiatric Genomics Consortium (PGC), Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium, 23andMe Research Team et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75.
Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes. 2018;42:1161–76.
Day FR, Helgason H, Chasman DI, Rose LM, Loh P-R, Scott RA, et al. Physical and neurobehavioral determinants of reproductive onset and success. Nat Genet. 2016;48:617–23.
Mills MC, Tropf FC, Brazel DM, van Zuydam N, Vaez A, eQTLGen Consortium et al. Identification of 371 genetic variants for age at first sex and birth linked to externalising behaviour. Nat Hum Behav. 2021;5:1717–30.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Justice AE, Winkler TW, Feitosa MF, Graff M, Fisher VA, Young K, et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat Commun. 2017;8:14977.
Yan X, Wang Z, Schmidt V, Gauert A, Willnow TE, Heinig M, et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol Metab. 2018;8:180–8.
Sanchez-Roige S, Fontanillas P, Jennings MV, Bianchi S, Huang Y, Hatoum A, et al. Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Mol Psychiatry. 2021;26:6209–17.
Sanchez-Roige S, Fontanillas P, Elson SL, the 23andme research team, Pandit A, Schmidt EM, et al. Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat Neurosci. 2018;21:16–8.
Cyders MA, Littlefield AK, Coffey S, Karyadi KA. Examination of a short English version of the UPPS-P impulsive behavior scale. Addict Behav. 2014;39:1372–6.
Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Personal Individ Differ. 2001;30:669–89.
Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. Wiley Period Inc. 1995;51:768–74.
VanderBroek L, Acker J, Palmer AA, de Wit H, MacKillop J. Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacol (Berl). 2016;233:39–48.
Durand EY, Do CB, Mountain JL, Macpherson JM. Ancestry composition: a novel, efficient pipeline for ancestry deconvolution. medrxiv. 2014.
Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993.
Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48:1031–6.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLOS Comput Biol. 2015;11:e1004219.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Verma A, Bradford Y, Dudek S, Lucas AM, Verma SS, Pendergrass SA, et al. A simulation study investigating power estimates in phenome-wide association studies. BMC Bioinforma. 2018;19:120.
Ehm MG, Aponte JL, Chiano MN, Yerges-Armstrong LM, Johnson T, Barker JN, et al. Phenome-wide association study using research participants’ self-reported data provides insight into the Th17 and IL-17 pathway. PLOS ONE. 2017;12:e0186405.
Lloyd KCK. A knockout mouse resource for the biomedical research community: the KOMP repository. Ann N. Y Acad Sci. 2011;1245:24–6.
Evangelou E, Gao H, Chu C, Ntritsos G, Blakeley P, Butts AR, et al. New alcohol-related genes suggest shared genetic mechanisms with neuropsychiatric disorders. Nat Hum Behav. 2019;3:950–61.
Ikeda M, Takahashi A, Kamatani Y, Momozawa Y, Saito T, Kondo K, et al. Genome-wide association study detected novel susceptibility genes for schizophrenia and shared trans-populations/diseases genetic effect. Schizophr Bull. 2019;45:824–34.
Howard DM, Adams MJ, Clarke T-K, Hafferty JD, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Yang H, Liu D, Zhao C, Feng B, Lu W, Yang X, et al. Mendelian randomization integrating GWAS and eQTL data revealed genes pleiotropically associated with major depressive disorder. Transl Psychiatry. 2021;11:225.
Baselmans BML, Jansen R, Ip HF, van Dongen J, et al. Multivariate genome-wide analyses of the well-being spectrum. Nat Genet. 2019;51:445–51.
Hill WD, Weiss A, Liewald DC, Davies G, Porteous DJ, Hayward C, et al. Genetic contributions to two special factors of neuroticism are associated with affluence, higher intelligence, better health, and longer life. Mol Psychiatry. 2020;25:3034–52.
Teixeira JR, Szeto RA, Carvalho VMA, Muotri AR, Papes F. Transcription factor 4 and its association with psychiatric disorders. Transl Psychiatry. 2021;11:19.
Van Esch H, Colnaghi R, Freson K, Starokadomskyy P, Zankl A, Backx L, et al. Defective DNA polymerase α-primase leads to X-linked intellectual disability associated with severe growth retardation, microcephaly, and hypogonadism. Am J Hum Genet. 2019;104:957–67.
Christakoudi S, Evangelou E, Riboli E, Tsilidis KK. GWAS of allometric body-shape indices in UK Biobank identifies loci suggesting associations with morphogenesis, organogenesis, adrenal cell renewal and cancer. Sci Rep. 2021;11:10688.
Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–74.
Nagel M, Watanabe K, Stringer S, Posthuma D, van der Sluis S. Item-level analyses reveal genetic heterogeneity in neuroticism. Nat Commun. 2018;9:905.
Lo M-T, Hinds DA, Tung JY, Franz C, Fan C-C, Wang Y, et al. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet. 2017;49:152–6.
23andMe Research Team, Thorp JG, Campos AI, Grotzinger AD, Gerring ZF, An J, et al. Symptom-level modeling unravels the shared genetic architecture of anxiety and depression. Nat Hum Behav. 2021;5:1432–42.
Feitosa MF, Kraja AT, Chasman DI, Sung YJ, Winkler TW, Ntalla I, et al. Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLOS ONE. 2018;13:e0198166.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–8.
Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun. 2019;10:339.
Hill WD, Davies NM, Ritchie SJ, Skene NG, Bryois J, Bell S, et al. Genome-wide analysis identifies molecular systems and 149 genetic loci associated with income. Nat Commun. 2019;10:5741.
Zhao B, Luo T, Li T, Li Y, et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat Genet. 2019;51:1637–44.
Gaddis N, Mathur R, Marks J, Zhou L, Quach B, Waldrop A, et al. Multi-trait genome-wide association study of opioid addiction: OPRM1 and Beyond. Addict Med. 2021;12:16873.
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–44.
Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. 2018;9:2098.
Rovira P, Demontis D, Sánchez-Mora C, Zayats T, et al. Shared genetic background between children and adults with attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2020;45:1617–26.
Pritikin JN, Neale MC, Prom-Wormley EC, Clark SL, Verhulst B. GW-SEM 2.0: efficient, flexible, and accessible multivariate GWAS. Behav Genet. 2021;51:343–57.
MacKillop J, Weafer J, Gray CJ, Oshri A, Palmer A, de Wit H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacol (Berl). 2016;233:3361–70.
Gray JC, MacKillop J, Weafer J, Hernandez KM, Gao J, Palmer AA, et al. Genetic analysis of impulsive personality traits: examination of a priori candidates and genome-wide variation. Psychiatry Res. 2018;259:398–404.
Leppert B, Millard LAC, Riglin L, Davey Smith G, Thapar A, Tilling K, et al. A cross-disorder PRS-pheWAS of 5 major psychiatric disorders in UK Biobank. PLOS Genet. 2020;16:e1008185.
Metha JA, Brian ML, Oberrauch S, Barnes SA, Featherby TJ, Bossaerts P, et al. Separating probability and reversal learning in a novel probabilistic reversal learning task for mice. Front Behav Neurosci. 2020;13:270.
Mota NR, Araujo-Jnr EV, Paixão-Côrtes VR, Bortolini MC, Bau CHD. Linking dopamine neurotransmission and neurogenesis: the evolutionary history of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster. Genet Mol Biol. 2012;35:912–8.
Greenbaum L, Ravona-Springer R, Livny A, Shelly S, Ganmore I, Alkelai A, et al. The CADM2 gene is associated with processing speed performance - evidence among elderly with type 2 diabetes. World J Biol Psychiatry. 2019;20:577–83.
Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94.
Collins RL, Glessner JT, Porcu E, Niestroj L-M, Ulirsch J, Kellaris G, et al. A cross-disorder dosage sensitivity map of the human genome. Cell. 2022;185:3041–55.e25.
Karlsson Linnér R, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51:245–57.
van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, et al. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014;39:3112–22.
Cope ZA, Halberstadt AL, van Enkhuizen J, Flynn AD, Breier M, Swerdlow NR, et al. Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: evidence from no-light and pharmacological challenges. Psychopharmacol (Berl). 2016;233:3513–25.
Hsu T-Y, Lee H-C, Lane TJ, Missal M. Temporal preparation, impulsivity and short-term memory in depression. Front Behav Neurosci. 2019;13:258.
Morris J. Genetic variation in CADM2 as a link between psychological traits and obesity. Sci Rep. 2019;9:7339.
Clarke T-K, Adams M, Davies G, Howard D, Hall L, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank. Mol Psychiatry. 2017;22:1376–84.
Kranzler HR, Zhou H, Kember RL, Smith RV, Justice AC, Damrauer S, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10:1499.
Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. The synaptic adhesion molecule SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology. 2013;38:628–38.
Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–31.
Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–30.
Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, et al. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron. 2010;68:894–906.
Yamada A, Inoue E, Deguchi-Tawarada M, Matsui C, Togawa A, Nakatani T, et al. Necl-2/CADM1 interacts with ErbB4 and regulates its activity in GABAergic neurons. Mol Cell Neurosci. 2013;56:234–43.
Niederkofler V, Baeriswyl T, Ott R, Stoeckli ET. Nectin-like molecules/SynCAMs are required for post-crossing commissural axon guidance. Development. 2010;137:427–35.
Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, et al. Nectin-like proteins mediate axon–Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–74.
Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, et al. A central role for Necl4 (SynCAM4) in Schwann cell–axon interaction and myelination. Nat Neurosci. 2007;10:861–9.
Park J, Liu B, Chen T, Li H, Hu X, Gao J, et al. Disruption of Nectin-Like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–9.
Enticott PG, Ogloff JRP, Bradshaw JL. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Personal Individ Differences. 2006;41:285–94.
Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: implications in substance abuse and dependence. Addict Disord Their Treat. 2003;2:33–40.
Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA. Genetic background limits generalizability of genotype-phenotype relationships. Neuron. 2016;91:1253–9.
Acknowledgements
We would like to thank the research participants and employees of 23andMe for making this work possible.
We would also like to thank The Externalizing Consortium for sharing the GWAS summary statistics of externalizing. The Externalizing Consortium: Principal Investigators: Danielle M. Dick, Philipp Koellinger, K. Paige Harden, Abraham A. Palmer. Lead Analysts: Richard Karlsson Linnér, Travis T. Mallard, Peter B. Barr, Sandra Sanchez-Roige. Significant Contributors: Irwin D. Waldman. The Externalizing Consortium has been supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA015416 -administrative supplement), and the National Institute on Drug Abuse (R01DA050721). Additional funding for investigator effort has been provided by K02AA018755, U10AA008401, P50AA022537, as well as a European Research Council Consolidator Grant (647648 EdGe to Koellinger). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above funding bodies. The Externalizing Consortium would like to thank the following groups for making the research possible: 23andMe, Add Health, Vanderbilt University Medical Center’s BioVU, Collaborative Study on the Genetics of Alcoholism (COGA), the Psychiatric Genomics Consortium’s Substance Use Disorders working group, UK10K Consortium, UK Biobank, and Philadelphia Neurodevelopmental Cohort.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
MVJ, SBB, YH, SSR and AAP were supported by funds from the California Tobacco-Related Disease Research Program (TRDRP; Grant Number 28IR-0070, T29KT0526 and T32IR5226). SBB was also supported by P50DA037844, SSR was also supported by NIH/NIDA DP1DA054394. JM and SSR were supported by the Brain and Behavior Foundation (grant 27676) and the Families for Borderline Personality Disorder Research (Beth and Rob Elliott) 2018 NARSAD Young Investigator Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SAB was supported by NIH/NIMH grants R01MH108653 and R21MH117518. AMBL was supported by NIH/NIAAA grant K99AA027835. TB acknowledges support by NIH/NIDA R01 DA018928. JM is supported by the Peter Boris Chair in Addictions Research. HHAT is funded through a Natural Science and Engineering Research Council PGS-D scholarship, and studies in Cohort 2 were supported by the Canadian Institutes of Health Research Project Grant (PJT-173442 to JYK). PF and SLE are employees of 23andMe, Inc., and hold stock or stock options in 23andMe. JY reports having received grant support funding from Sunovion, Heptares, and Gilgamesh, as well as honoraria from Marvel Biotech, none of which were involved in the current project. The other authors report no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanchez-Roige, S., Jennings, M.V., Thorpe, H.H.A. et al. CADM2 is implicated in impulsive personality and numerous other traits by genome- and phenome-wide association studies in humans and mice. Transl Psychiatry 13, 167 (2023). https://doi.org/10.1038/s41398-023-02453-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02453-y
This article is cited by
-
Genetic influences and causal pathways shared between cannabis use disorder and other substance use traits
Molecular Psychiatry (2024)
-
Pleiotropy and genetically inferred causality linking multisite chronic pain to substance use disorders
Molecular Psychiatry (2024)
-
Genetic Influences on Alcohol Sensitivity: a Critical Review
Current Addiction Reports (2023)