Abstract
Rates of return to use in addiction treatment remain high. We argue that the development of improved treatment options will require advanced understanding of individual heterogeneity in Substance Use Disorders (SUDs). We hypothesized that considerable individual differences exist in the three functional domains underlying addiction—approach-related behavior, executive function, and negative emotionality. We included N = 593 participants from the enhanced Nathan Kline Institute-Rockland Sample community sample (ages 18–59, 67% female) that included N = 420 Controls and N = 173 with past SUDs [54% female; N = 75 Alcohol Use Disorder (AUD) only, N = 30 Cannabis Use Disorder (CUD) only, and N = 68 Multiple SUDs]. To test our a priori hypothesis that distinct neuro-behavioral subtypes exist within individuals with past SUDs, we conducted a latent profile analysis with all available phenotypic data as input (74 subscales from 18 measures), and then characterized resting-state brain function for each discovered subtype. Three subtypes with distinct neurobehavioral profiles were recovered (p < 0.05, Cohen’s D: 0.4–2.8): a “Reward type” with higher approach-related behavior (N = 69); a “Cognitive type” with lower executive function (N = 70); and a “Relief type” with high negative emotionality (N = 34). For those in the Reward type, substance use mapped onto resting-state connectivity in the Value/Reward, Ventral-Frontoparietal and Salience networks; for the Cognitive type in the Auditory, Parietal Association, Frontoparietal and Salience networks; and for the Relief type in the Parietal Association, Higher Visual and Salience networks (pFDR < 0.05). Subtypes were equally distributed amongst individuals with different primary SUDs (χ2 = 4.71, p = 0.32) and gender (χ2 = 3.44, p = 0.18). Results support functionally derived subtypes, demonstrating considerable individual heterogeneity in the multi-dimensional impairments in addiction. This confirms the need for mechanism-based subtyping to inform the development of personalized addiction medicine approaches.
Similar content being viewed by others
Introduction
In the United States, substance use disorders (SUDs) are a major source of morbidity and mortality. The lifetime prevalence of SUDs has been estimated to be as high as 30% [1]. Currently, approximately 40.3 million people in the U.S. have a SUD diagnosis [2]. Independent of their primary substance of choice, over two-thirds of individuals return to use within weeks to months of initiating treatment, and up to 85% of individuals return to substance use within one year of treatment completion [2,3,4]. Research suggests that this is due to suboptimal treatment options, low confidence in their effectiveness, and restricted access to treatment facilities [5, 6]. We argue that alleviating this urgent situation; specifically, improving the effectiveness of existing treatments, will require a better understanding of the mechanisms underlying the vulnerability for SUDs to develop and to persist. Furthermore, we recognize that the underlying neurobehavioral mechanisms are likely to be heterogeneous across individuals with SUDs, and understanding this heterogeneity will be critical for conceptualizing advances in treatment.
Prominent addiction theories have stressed the importance of three neurobehavioral mechanisms of persistence in addiction: (1) altered incentive salience (also reward- or approach-related behavior), (2) lower executive function, and (3) increased negative emotionality [7, 8]. Recent evidence from individuals with alcohol use disorder (AUD) has empirically demonstrated the validity of this three-mechanism model of addiction [9, 10]. It has further been argued that these three mechanisms are dependent on each other, going back to studies showing that substances of abuse impair all of the neurobiological systems underlying them [11,12,13,14]. However, contrary to this assumption, decades-old research from psychology provided evidence for the functional independence of the approach-related behavior, executive function and negative emotionality domains [15, 16]. Accordingly, we propose to investigate if function on these three domains is at least partially independent, such that different combinations of impairments on these domains may underlie addiction persistence.
Efforts to investigate heterogeneity and identify subtypes within populations with SUDs have largely focused on clinical/diagnostic data that assessed addiction severity (and sometimes psychiatric comorbidity), rather than the underlying mechanisms [17,18,19,20,21,22,23]. Through literature searches (PubMed, Google Scholar), we have identified over 120 published studies that have subtyped individuals with SUDs using symptom scales (e.g., AUDIT, ASI, AUDADIS-IV, SSADDA, DSM-IV, ICD-10Footnote 1). These initial efforts to define subgroups or “subtypes” in individuals with SUDs consistently found that substance users can be separated into a milder “late onset” type, consisting of individuals characterized by low psychiatric comorbidity versus a more severe “early onset” type with high psychiatric comorbidity, more poly-substance use, and worse treatment prognosis [24,25,26,27,28,29,30,31,32,33]. In contrast, previous research characterizing individual differences in the multi-dimensional mechanisms (rather than symptom presentation) of addiction is extremely limited. Only two studies have investigated heterogeneity in impulsivity and found “impulsive” versus “non-impulsive” types [34, 35], while two other studies differentiated individuals based on drinking motives and found a “reward” (pleasure seeking) versus “relief” (drinking to cope) motivational type [36, 37]. Thus, even within the space of mechanism-based subtyping, existing literature is limited to a narrowly-focused space of the mechanisms underlying addiction, rather than applying a broad, multi-dimensional approach.
The goal of the current study was to use a multi-dimensional “mechanism-based” subtyping approach, using data informing the three functional domains implicated in addiction persistence: (1) approach-related behavior, (2) executive function, and (3) negative emotionality [7, 8]. We leveraged the enhanced Nathan Kline Institute-Rockland Sample (NKI-RS), a dataset which includes a comprehensive phenotypic assessment that broadly characterized individual function on these three domains, provided a clinical characterization of the sample, and included neuroimaging data. This large, heterogeneous community sample included participants with varying past SUD diagnoses. We hypothesized considerable individual heterogeneity in the impairments on the three functional domains of interest. We further hypothesized that individual heterogeneity underlying addiction would have a large trait-component. We hence expected to find the existence of at least three separable neuro-behaviorally distinct subtypes in past substance users: a “reward type” with higher reward-seeking, a “cognitive type” with impaired executive function, and a “relief type” defined by the use of substance to cope with high negative affect. Finally, we expected that each discovered subtype would be characterized by corresponding, unique neurobiological impairments of resting-state brain function.
Participants and methods
Participants
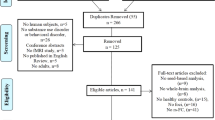
We analyzed data from the NKI-RS study that was collected at the NKI in New York, from 2012 to 2016 [38]. All study procedures and informed consent forms, including consent to share de-identified data, were approved by the Nathan Kline Institute Institutional Review Board (IRB) in accordance with the Declaration of Helsinki. After removing measures with >10% missing data, N = 612 participants ages 18–59 had complete phenotypic data. Of these participants, N = 420 were Controls (participants with neither a past nor a current SUD diagnosis), N = 19 had current SUDs, and N = 173 were participants with past SUDs (54% females). Due to their low number, individuals with current SUDs were excluded, leaving N = 593 participants (67% female) in the sample. Within individuals with past SUDs, N = 75 were diagnosed with past AUD only, N = 30 were diagnosed with past Cannabis Use Disorder (CUD) only and N = 68 were diagnosed with Multiple SUD diagnoses, see Supplementary Table 1. Detailed demographic information is provided in Table 1a. All clinical diagnoses (SUD and comorbid diagnoses) were assessed with the Structured Clinical Interview for DSM-IV [39]. Tobacco Use Disorder was not assessed, due to the low predictive validity of this diagnosis [40].
Phenotypic measures
The NKI-RS data set phenotyped participants using self-report measures and tasks that assessed behavior, affect, clinical symptoms and cognition. We included all available 74 subscales (derived from 18 different measures) that had <10% missing data (listed in Supplementary Table 2). We did therefore not perform any a priori selection of measures based on theoretical considerations, but rather intended to model the entire phenotypic space.
Factor analysis of phenotypic data
To reduce the measured phenotypic space to a set of latent constructs, an exploratory factor analysis (EFA) was conducted that included all study participants (N = 593) to model the full range of phenotypic variance. This analysis was very well powered (N > 500; participant/variable ratio=8.0), based on sample size recommendations for EFA [41]. We used Monte Carlo permutation analysis (parallel analysis) [42] to determine the number of factors statistically significant at p < 0.05 [43]. Factors were extracted using maximum likelihood as calculated by the expectation-maximization algorithm. Additionally, an oblimin rotation was used to allow for correlated factors. The use of oblimin rotation is critical to data reduction over a large phenotypic variable space, as many factors are expected to be separable but closely related. EFA was conducted in R using the “psych” package [44]. Details on why this method was chosen over other data reduction schemes are included in the Supplementary Methods. One participant (N = 1) was deemed an outlier (>3 SD from mean) on their factor loading for “general psychiatric symptoms” and was removed from further analysis.
Extraction of functional domains
To visualize how latent constructs (factors generated by the EFA) were related to each other, we used a spring-embedded plot. Each factor was visualized as a node. Edges connecting nodes act as “springs” between pairs of nodes. Nodes (factors) that were more strongly correlated were plotted closer together in two-dimensional space, to provide an intuitive visual representation of the factor correlations [45]. The algorithm was run on the full correlation matrix from the EFA, using MATLAB’s “graph” object. Only minimal correlation weights were used, such that each factor was connected to at least one other factor.
Subtyping analysis in past SUDs
To recover distinct subgroups within individuals with past SUD diagnosis (N = 173), subtypes were determined using latent profile analysis (LPA) on the EFA latent factors scores. LPA, a form of Gaussian-mixture modeling, builds a model of the data to find subtypes within the modeled multi-dimensional data variable space [46], which here is the variable space defined by the EFA. LPA was performed in R (4.0.5) using the “mclust” package (version 5.7.4) [47, 48]. The model was initialized by model-based hierarchical clustering, and an expectation-maximization algorithm was used to fit the model by assigning posterior probabilities to variable distributions [49, 50]. Bayesian Information Criteria (BIC) were computed for each parameterized model given the log-likelihood, the dimension of data, and the number of mixture components in the model. The model with the lowest BIC and non-negative BIC difference was selected [51]. The bootstrap likelihood ratio test (BLRT) was also used to evaluate model fit to models with k−1 profiles [52]. The power for this analysis (N = 173; 12 input variables that are continuous; N = 34 in the smallest observed class; observed effect size for profile differentiation: d = 1.7–2.8) was excellent (close to 1) according to simulations [51, 53].
Phenotypic characterization of recovered subtypes
To characterize the recovered subtypes with regards to their phenotypic profiles, we tested for statistically significant differences between the recovered types on the extracted EFA factors using independent samples t test with Holm–Bonferroni correction. We also correlated individual EFA factor scores with current non-medicinal substance use (number of days in the past 6 months) and binge-drinking (number of days in the past 6 months; men: 5 or more drinks per day, women: 4 or more drinks per day) with Holm-Bonferroni correction.
Resting-state functional connectivity per subtype
To characterize resting-state brain function underlying return to substance use and to investigate if the neural correlates of return to use would differ between subtypes, we conducted a resting-state functional connectivity analysis of the neural correlates of continued substance use for each subtype separately. We included the subset of participants with complete NIDA Quick Screen and Adult Self Report (ASR) data and more than 5 min of resting-state data after denoising (Past SUD N = 104, 59% Female; Controls N = 302, 74% Female). We used the matchControls function from the R “e1071” library [54] to determine a separate age- and gender-matched control group for each subtype. We also assessed urine screens on MRI day (Supplementary Table 3).
Imaging data was acquired on a Siemens Trio 3.0 T scanner (Siemens Healthcare GmbH, Erlangen, Germany) equipped with a 32-channel head coil [38]. For each participant, a T1-weighted (T1w) magnetization prepared gradient echo sequence was acquired for a structural image (repetition time=1900 ms, echo time=2.52 ms, flip angle=9°, 176 slices, 1 mm3 isotropic voxels). To analyze resting-state functional connectivity, we used the “REST645” scan that was acquired using a multiband echo-planar imaging sequence with the following parameters: multiband factor=4, volumes=900, repetition time=645 ms, echo time=30 ms, flip angle=60°, 3 mm3 isotropic voxels.
For data quality control, MRI Quality Control tool (MRIQC) was implemented prior to fMRI data pre-processing [55]. Scans were entered through a minimal preprocessing pipeline to obtain images and masks for Image Quality Metrics (IQMs): signal-to-noise ratio, framewise-displacement (FD), and segmented tissue summary statistics. The data from outliers on these metrics (>3 SD) were assessed visually. All scans were of high quality.
fMRI preprocessing was performed using fMRIPrep v. 20.2.1 [56]. The T1w image, which was preprocessed as described in the Supplementary Methods, was used for spatial normalization and alignment. For each BOLD-run per subject, the following preprocessing was performed. First, the initial first four volumes were non-steady state scans and were removed. Then, a reference volume and its skull-stripped version were generated and corrected for susceptibility distortions using fMRIPrep’s fieldmap-less approach. The BOLD reference was then co-registered to the T1w reference using bbregister (FreeSurfer v6.0.1) which implements boundary-based registration [57]. Co-registration of the BOLD reference to the T1 reference was configured with nine degrees of freedom to account for distortions remaining in the BOLD reference. Head-motion parameters with respect to the BOLD reference (transformation matrices and size rotation and translation parameters) for later denoising were estimated using MCFLIRT (FSL 5.0.9 [58]) before any spatiotemporal filtering. Head motion correction was performed in the original, native space. The BOLD time series were resampled onto the FreeSurfer surface: “fsaverage” and then into standard space (MNI152NLin2009cAsym). Grayordinates files containing 91k samples were generated.
Next, denoising of the data was performed in Matlab (R2019a) using custom scripts to regress out the following noise regressors: five aCompCor principal components from both the segmented WM and CSF [59], 24 motion parameters (3 rotation, 3 translation, their derivatives and quadratic terms [60]; cosine filters (128 s cut off)—low-frequency signal drift—regressors, and spike regressors for each frame that exceeded a threshold of 0.5 mm FD [61, 62].
For functional connectivity analyses, we used Glasser et. al.’s 2016 multimodal parcellation (360 cortical areas [63]), in addition to the 19 anatomically defined subcortical areas from the HCP [64]. We constructed functional connectivity matrices by extracting the mean BOLD time series for these 379 areas and subsequently computing the z-transformed Pearson correlation coefficients between the time-courses of all areas. The resulting matrices were binarized with a proportional threshold of 15%, which is the median of an ideal cost range (approx. 0.01–0.30). This provides for optimized sparsity in the thresholded graph [65] and improved stability of measures as compared to absolute thresholds [66].
We used a graph theory approach to calculate nodal global efficiency, local efficiency, betweenness centrality, and participation coefficient using the Brain Connectivity Toolbox [67]. We used three measures of functional integration and segregation—nodal global efficiency, local efficiency, and betweenness centrality. The efficiency metrics, which are known to be powerful and biologically plausible measures of brain network function, quantify the shortest path length between a select node and all other nodes in the brain (global) or neighboring nodes (local), whereas the betweenness measure quantifies the number of paths that pass through a given node and thus assesses that node’s criticality in the network [67, 68]. Additionally, to assess centrality, we used the graph theory measure participation coefficient, which quantifies whether a node is facilitating modular segregation [67, 69]. Module assignments for computing participation coefficient were chosen a priori based on the same multimodal parcellation as used to define areas, [63] assigning each of Glasser et al.’s 22 regions and the 19 anatomically defined subcortical areas as a module. See Supplementary Methods for more details.
For each graph theory metric, a generalized linear model (GLM) regression analysis was run and corrected for multiple comparisons using an FDR-threshold of p < 0.05. Separate analyses were performed to investigate the subtype-specific neurobiological correlates of current non-medicinal substance use (predictor: number of days in the past 6 months) and current binge-drinking (predictor: number of days in the past 6 months; men: 5 or more drinks per day, women: 4 or more drinks per day). Results were visualized using Connectome Workbench (version 1.5.0) (https://www.humanconnectome.org/software/connectome-workbench [70]). To aid the discussion and interpretation of results, the 360 cortical areas were additionally assigned to large-scale resting-state networks by first matching each area to its Brodmann Area and then determining the corresponding Intrinsic Connectivity Network based on Laird et al. (Supplementary Fig. 1) [71].
Results
Phenotypic space
The Bartlett’s test of Sphericity (X2 = 21299.81, p < 0.001) and Kaiser–Meyer–Olkin (KMO) test (KMO = 0.86) indicated appropriateness of the phenotypic data for EFA. Across all participants, we extracted 12 latent factors (p < 0.05), thus reducing the 74-variable phenotypic space into a 12-dimensional latent phenotypic space, within which we then performed the subsequent subtyping analysis. The 12 extracted latent factors collectively accounted for 44.8% of the common variance (see Supplementary Fig. 2 for the scree plot from the parallel analysis and Supplementary Table 4 for the EFA results). EFA model fit indices indicated good factor separation (RMSEA = 0.046, Tucker-Lewis Index = 0.81) and each factor had the minimum requirement of three salient loadings each [72, 73]. The 12 factors, their labels and factor loadings are displayed in Supplementary Table 5.
From the 12 extracted latent factors, three factors saliently (>|0.30|) loaded on both trait and state variables (internalizing, effortful control, extraversion/sociability), one factor saliently loaded only on variables derived from performed tasks (executive function), one factor saliently loaded only on state variables (general psychiatric symptoms) and the remaining seven factors saliently loaded only on trait variables (Supplementary Table 5). The spring-embedded plot derived from the underlying factor correlations demonstrated that the twelve latent factors self-organized into three hypothesized phenotypic functional domains (Fig. 1, see Supplementary Table 6 for the factor correlations). These domains were: (1) an approach-related behavior domain that included social risk-taking, unethical behavior, sensation seeking, and risk perception; (2) an executive function domain that consisted of the factors executive function and openness/sensitivity; and (3) a negative emotionality domain that encompassed internalizing, general psychiatric symptoms, urgency, negative affect (trait), and (lack of) effortful control, see Fig. 1.
In a spring-embedded network, nodes that are more strongly correlated are plotted closer together in two-dimensional space. Each node represents an EFA factor. Three functional domains emerged across all participants based on the underlying factor correlations: (1) an Approach-Related functional domain (green), (2) an Executive Function functional domain (blue), and (3) a Negative Emotionality functional domain (red).
Recovered subtypes
The LPA performed within this 12-dimensional phenotypic space and within participants with past SUD found that three subtypes existed among those with past SUD. BLRT was significant up to a 5-cluster solution (pFDR < 0.05), however, the 3-cluster solution was chosen as the best model based on how the 3-cluster solution exceeded the second-best model of a 4-cluster solution (delta-BIC = 7.32). Demographic characteristics, clinical and substance use data for the recovered subtypes are described in Supplementary Tables 7a, b. The subtypes differed from each other in their phenotypic profiles (p < 0.05, Supplementary Table 8), with very large effect sizes for differences in the factors that best differentiated between subtypes (Cohen’s D = 1.7–2.8; Supplementary Table 9). We found (1) a “Reward type” with higher sensation-seeking, social risk-taking, and unethical behavior; (2) a “Cognitive type” with lower openness/sensitivity and lower performance on executive function tasks; (3) and a “Relief type” with high internalizing and general psychiatric symptoms and higher negative affect (trait) (see Fig. 2 for the phenotypic profiles of these subtypes). Additional results confirmed that we found the same subtype-specific profiles when comparing each subtype to control participants (see Supplementary Table 10). Importantly, while these results confirmed that impairments on all three hypothesized domains are linked to addiction, each discovered SUD subtype was characterized by impairments on only one of the three domains.
Subtypes were determined using latent profile analysis on the EFA latent factors scores within individuals with past SUD diagnosis (N = 173). Three subtypes were recovered, which included a Reward Type with higher sensation seeking, social risk-taking, and unethical behavior (green); a Cognitive Type with lower openness/sensitivity and a lower executive function (blue), and a Relief Type with higher internalizing, general psychiatric symptoms, and negative affect (trait) (red). Independent samples t-test were done to compare the subtypes statistically (p < 0.05*, p < 0.01**). Error bars are standard error.
Demographic and clinical data converged with these results such that the Reward type had significantly higher non-medicinal substance use (past 6 months) and greater frequency of urine screens positive for tetrahydrocannabinol (THC) on MRI day, the Cognitive type had lower levels of education, and the Relief type had a significantly higher rate of diagnosed internalizing disorders (Supplementary Table 7a). While none of the correlations between EFA scores and current (modest) substance use were significant after Holm-Bonferroni correction, before correction the Reward type showed a correlation of current non-medicinal substance use with unethical behavior (r = 0.24, p = 0.05), and the Cognitive type with effortful control (r = 0.32, p < 0.05). Finally, there was no difference in the distribution of past primary SUD diagnosis (equal distribution, χ2 = 4.71, p = 0.32) or gender distribution (χ2 = 3.44 p = 0.18) between the three recovered subtypes.
Resting-state functional connectivity per subtype
Each subtype demonstrated unique functional connectivity patterns that were linked to current return to substance use (see Table 2). For individuals in the Reward type, non-medicinal substance use was associated with functional connectivity in the Value/Reward (ACC/mPFC), Ventral Frontoparietal (inferior frontal), and the Salience (insula, frontal opercular, ACC/mPFC) networks. For individuals in the Cognitive type, non-medicinal substance use was linked to functional connectivity in the Auditory (auditory association cortex), Parietal Association (paracentral lobular/mid-cingulate), Frontoparietal (superior parietal), and Salience (ACC/mPFC) networks. For individuals in the Relief type, non-medicinal substance use was associated with functional connectivity with non-medicinal substance use in the Parietal Association (paracentral lobular/mid-cingulate), higher visual (motion complex, neighboring visual cortex) and Salience (paracentral lobular/mid-cingulate, insular, frontal opercular) networks. Detailed results including the specific implicated areas, regions, affected graph theory measures, directions of effects, and FDR-corrected p values for each significant parcel are summarized in Table 2. To visualize results, areas were projected onto template brains by subtype, collapsing across graph theory metrics in Supplementary Fig. 3. These results converged partially with the analysis on resting-state functional connectivity alterations related to alcohol binge drinking (see Supplementary Table 11 for detailed results). In the Relief type, three networks (Higher Visual, Salience, Auditory) were linked to both binge drinking and non-medicinal substance use. Both Relief and Reward type uniquely demonstrated an involvement of the Motor Planning networks linked to binge drinking, but not non-medicinal substance use. There were no significant results for the Cognitive type related to binge drinking.
Discussion
The goal of this study was to characterize individual heterogeneity across three functional domains in individuals with past SUDs. Our results revealed three distinct addiction subtypes, with each subtype demonstrating impairments in only one of the three relevant functional domains. For individuals in the Reward type, who showed higher approach-related behavior (e.g., high sensation seeking and the highest current non-medicinal substance use), substance use was related to functional connectivity in networks underlying value and reward representation. For individuals in the Cognitive type, who demonstrated challenges in executive function (e.g., lower executive function performance and lower levels of education), substance use was related to functional connectivity in networks underlying cognition, such as the Frontoparietal network. For individuals in the Relief type, who demonstrated higher negative emotionality (e.g., more internalizing and psychiatric symptoms, and more frequent diagnosis of internalizing disorders), substance use was related to functional connectivity in visual association networks. Overall, these results support the existence of substantial individual differences in the three mechanisms that have been linked to addiction. Importantly, while previous work had revealed abundant evidence on heterogeneity in approach/reward behavior, executive function and negative emotionality in addiction by investigating each functional domain separately [74,75,76,77,78,79,80,81,82], this is the first study to show that there are subgroups with differential functional profiles across these three domains.
The first step of our analysis provided empirical evidence for the existence of a general phenotypic space with three separable functional domains: 1) approach-related behavior, 2) executive function, and 3) negative emotionality. These results therefore support previous research proposing these as three independent (separable) behavioral domains [15, 16, 83], which ultimately led to the conceptualization of these as Research Domain Criteria (RDoC) domains [84, 85]. Importantly, our results further provide empirical evidence for the equal relevance of all three functional domains in addiction. This is important, since consensus discussions on the relevance of the RDoC domains for explaining addiction have mainly focused on constructs from the approach-related “positive valence” domain (e.g., “reward valuation,” “reward learning”, “expectancy”, “action selection”, and “habit”) [86]. Our results, however, support the equal importance of the RDoC cognitive and negative valence systems in SUDs, in accordance with a wealth of research demonstrating the importance of lower executive function and increased negative emotionality as addiction persistence mechanisms [7, 8, 87].
Our results further expand on previous work on functional domains in AUD in several ways. First, we provide additional empirical evidence for the relevance of three data-derived functional domains shown to be relevant in AUD by Kwako and colleagues: (1) incentive salience, (2) executive function, and (3) negative emotionality [10, 88]. Kwako and colleagues used a similar approach as ours for deriving the functional domains (EFA), in a data set that included 43.5% individuals with AUD. Overall, they found functional domains that were more narrowly defined than ours, largely due to differences in the included measures. Kwako and colleagues selected measures based on their theoretical relevance to addiction models (personal correspondence with Dr. Kwako), whereas we used an agnostic approach and included all available phenotypic data. Therefore, their “incentive salience” domain was indexed by the self-reported drive to consume substances, whereas our approach-/reward behavior domain was defined by the broader constructs of reward seeking (e.g., sensation seeking and social risk-taking). And while their executive function domain included measures of impulsivity, conscientiousness, and perseverance, ours was indexed by the broader measures of openness/sensitivity and executive function, which loaded on tasks that assessed cognitive flexibility/shifting, attention shifting, planning and working memory [89,90,91]. Both their and our negative emotionality domains were very similar and included measures of negative affect, internalizing psychiatric symptoms and aggression. Further, in our analysis “effortful control” (e.g., lack of conscientiousness, and perseverance) was highly correlated to measures of negative emotionality, but not to the other measures of executive function, a finding which we have previously reported in a completely independent data set [92]. Overall, while our phenotypic space described a broader phenotypic space, our results align with Kwako’s empirically derived results, and hence provide additional empirical evidence for common addiction models (e.g., [8]) that propose the relevance of these three domains in addiction. Importantly, however, our results provide additional novel empirical evidence on the relevance of this framework beyond AUD, by demonstrating the existence of the same subtypes, with impairments in these three functional domains, independent of the primary substance of choice.
We found individual differences for both trait and state factors in our analysis. Each subtype was characterized by factors that assessed general, temperament or personality characteristics. Individuals in the Relief and Cognitive types were additionally characterized by the internalizing, general psychiatric and executive function factors, which loaded on behavioral tasks and self-report of current psychiatric symptom. Previous research has repeatedly shown a convergence between state and trait characteristics [93, 94]. Trait-like stable characteristics such as for example “anxious temperament” are highly correlated with and predictive of changes in psychiatric symptoms (e.g., anxiety, depression) over time [93, 94]. Executive function is influenced by a highly heritable (99%) common factor [95]. Both previous research and our current findings therefore support our hypothesis that there are significant stable, “trait-like” individual differences in addiction that are linked to differences in state variables and would hence be relevant in different phases such as onset, persistence, and recovery from addiction. We propose that our “trait-like” subtypes have clinical relevance, because these trait-like individual differences interact with more dynamic processes, such as for example gradual effects caused by chronic drug-use. For example, an individual with trait-level challenges in executive function would be more vulnerable to the additional loss of cognitive control induced by chronic drug use over time than an individual who does not have such trait-level challenges.
Our findings on subtype-specific brain function during resting-state mirrored our behavioral results. We found that each subtype demonstrated unique patterns of large-scale resting-state network connectivity underlying current substance use. Neural correlates of substance use were identified in the Value/Reward, Ventral Frontoparietal, and Salience networks for those in the Reward type. These three networks represent incentive motivational value [96], are involved in approach behavior [97,98,99], and allocation of attentional control [100, 101], in line with the greater approach-behavior observed in this subtype. Aberrant engagement of these networks has also repeatedly been shown to be correlated with craving and substance use frequency in SUDs [102, 103]. These findings may also reflect current non-medicinal substance use and greater frequency of positive THC urine screen on MRI day. For those in the Cognitive type, higher non-medicinal substance use mapped on to connectivity in the Auditory, Parietal Association, Frontoparietal, and Salience networks. The auditory network has been reported to be associated with cognitive impairment [104]. Furthermore, the Parietal Association, Frontoparietal and Salience networks have shown connectivity responses related to executive control dysfunction in addiction [103, 105, 106], converging with the finding of challenges in executive function observed in this subtype. For those in the Relief type, non-medicinal substance use and binge drinking mapped on to connectivity in Parietal Association, higher visual and Salience networks. These three networks are implicated in effortful control [12, 103, 107,108,109], have been shown to be hyper-engaged in those with higher levels of increased anxiety [109], and have greater connectivity due to “hyperscanning” of the environment [110, 111]. In summary, our analysis of resting-state brain function correlated with current non-medicinal substance use recovered distinct neurobiological features that fit the phenotypic profiles of each subtype.
It has been suggested—and we agree—that a better understanding of the substantial neural and behavioral heterogeneity within SUDs by means of subtyping could spur the development of personalized treatments and preventions, improve treatment effectiveness, and inform resource allocation [112,113,114,115,116]. Our results underscore the importance of this effort, by providing empirical evidence for substantial individual differences in the neurobehavioral impairments on three functional domains linked to addiction that can be targeted separately in a Personalized Medicine approach. Initial evidence on the improved efficacy of subtype-specific treatments comes from a series of studies in individuals with AUD. These studies demonstrated that a pharmacological treatment that blocks the brain’s “pleasure response” was efficacious only in a subgroup of patients that indicated that their main drinking motive was pleasure seeking, but was less effective in reducing drinking in individuals who drank to cope with negative affect [36, 112, 117]. Other efficacious subtype-specific targeted treatments for the Relief Type may be therapies that target emotion regulation [e.g., pharmacotherapies used in anxiety/depression [118]; cognitive reappraisal [109]; specialized cognitive-behavioral therapy for SUDs with comorbid mental health disorders [119]. In contrast, the Cognitive Type may be best treated with cognitive trainings [e.g., working memory training [120, 121], pharmacological cognitive enhancers [120] or neuromodulation therapies targeted at enhancing cognitive control [122, 123]. Finally, the Reward type may have the best outcomes with motivational approaches that refocus their reward-seeking to non-substance related and non-immediate rewards [124, 125]. In summary, there is increasing evidence that targeted treatments for SUDs indeed outperform non-targeted treatment options [36, 117, 119].
The current study is only an initial step into the investigation of subtypes in addiction. Future work refining the proposed subtypes and developing clinical tools to “diagnose” them is needed. A limitation of the current study is that participants with currently diagnosed SUD(s) were not included. Another limitation is that the data set did not include detailed clinical data, or follow-up clinical data. It was therefore not possible to directly link the observed individual differences to clinical outcomes within this study, though there is an abundance of previous research in clinical samples that have demonstrated the importance of individual differences in approach-/reward related behavior, executive function, and negative emotionality for clinical outcomes [4, 126,127,128,129].
Conclusion
As hypothesized, the current results provide empirical evidence for substantial individual differences in the mechanisms underlying addiction, and the existence of “trait-like” subtypes that each had impairments on only one of three identified addiction-relevant functional domains. These subtypes were found to be “addiction-general,” as they were independent of the different primary substances of choice. These results call for updated addiction models that factor in how these trait-like individual differences interact with the mechanisms driving the escalation and persistence of substance use. Finally, if further substantiated, these results could guide the development of individualized behavioral, pharmacological, and brain-focused preventative and treatment approaches that take these individual differences into account.
Data availability
All neuroimaging data are available for download through Amazon Web Services or NITRC and a data usage agreement is not required. Phenotypic data are available for download through the Longitudinal Online Research and Imaging System (LORIS) database and Collaborative Informatics and Neuroimaging Suite (COINS) databases upon approval of a data usage agreement with NKI. Details are provided at http://fcon_1000.projects.nitrc.org/indi/enhanced/access.html.
Notes
Abbreviations: AUDIT Alcohol Use Disorders Identification Test, ASI Addiction Severity Index, AUDADIS-IV Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV, SSADDA Semi-Structured Assessment for Drug Dependence and Alcoholism, DSM-IV Diagnostic and Statistical Manual of Mental Disorders-IV, ICD-10 International Statistical Classification of Diseases and Related Health Problems (10th revision).
References
Grant BF, Goldstein RB, Saha TD, Chou S, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. https://doi.org/10.1001/jamapsychiatry.2015.0584.
SAMHSA. Key Substance Use and Mental Health Indicators in the United States: results from the 2020 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. 2021. https://www.samhsa.gov/data/.
Kadam M, Sinha A, Nimkar S, Matcheswalla Y, De Sousa A. A comparative study of factors associated with relapse in alcohol dependence and opioid dependence. Indian J Psychol Med. 2017;39:627–33. https://doi.org/10.4103/IJPSYM.IJPSYM_356_17.
Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. https://doi.org/10.1007/s11920-011-0224-0.
Maruti S, Desjardins I, Bagge CL, Althoff RR. Commentary: Opioid use disorder and suicide: an important opportunity to address two significant public health epidemics. Prev Med. 2019;128:105854. https://doi.org/10.1016/j.ypmed.2019.105854.
Rapp RC, Xu J, Carr CA, Lane DT, Wang J, Carlson R. Treatment barriers identified by substance abusers assessed at a centralized intake unit. J Subst Abus Treat. 2006;30:227–35. https://doi.org/10.1016/j.jsat.2006.01.002.
Bickel WK, Mellis AM, Snider SE, Athamneh LN, Stein JS, Pope DA. 21st century neurobehavioral theories of decision making in addiction: review and evaluation. Pharm Biochem Behav. 2018;164:4–21. https://doi.org/10.1016/j.pbb.2017.09.009.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. https://doi.org/10.1016/S2215-0366(16)00104-8.
DeMartini KS, Gueorguieva R, Pearlson G, Krishnan-Sarin S, Anticevic A, Ji L, et al. Mapping data-driven individualized neurobehavioral phenotypes in heavy alcohol drinkers. Alcohol Clin Exp Res. 2021;45:841–53. https://doi.org/10.1111/acer.14580.
Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, et al. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. Am J Psychiatry. 2019;176:744–53. https://doi.org/10.1176/appi.ajp.2018.18030357.
Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. https://doi.org/10.3758/BF03199951.
Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. https://doi.org/10.1007/PL00005483.
Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001;24:97–129. https://doi.org/10.1016/S0893-133X(00)00195-0.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. https://doi.org/10.1016/0165-0173(93)90013-p.
Tellegen A, Waller NG. Exploring personality through test construction: development of the multidimensional personality questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. The SAGE handbook of personality theory and assessment: volume 2 - personality measurement and testing. Thousand Oaks, CA: SAGE Publications Inc.; 2008. pp. 261–92.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. https://doi.org/10.1037//0022-3514.54.6.1063.
Bucholz KK, Heath AC, Reich T, Hesselbrock VM, Kramer JR, Nurnberger JI, et al. Can we subtype alcoholism? A latent class analysis of data from relatives of alcoholics in a multicenter family study of alcoholism. Alcohol Clin Exp Res. 1996;20:1462–71. https://doi.org/10.1111/j.1530-0277.1996.tb01150.x.
Hedden SL, Martins SS, Malcolm RJ, Floyd L, Cavanaugh CE, Latimer WW. Patterns of illegal drug use among an adult alcohol dependent population: results from the National Survey on Drug Use and Health. Drug Alcohol Depend. 2010;106:119–25. https://doi.org/10.1016/j.drugalcdep.2009.08.002.
Horváth Z, Paksi B, Felvinczi K, Griffiths MD, Demetrovics Z, Urbán R. An empirically based typology of alcohol users in a community sample using latent class analysis. Eur Addict Res. 2019;25:293–302. https://doi.org/10.1159/000501516.
Kranzler HR, Wilcox M, Weiss RD, Brady K, Hesselbrock V, Rounsaville B, et al. The validity of cocaine dependence subtypes. Addict Behav. 2008;33:41–53. https://doi.org/10.1016/j.addbeh.2007.05.011.
Lynskey MT, Agrawal A, Bucholz KK, Nelson EC, Madden PAF, Todorov AA, et al. Subtypes of illicit drug users: a latent class analysis of data from an Australian twin sample. Twin Res Hum Genet. 2006;9:523–30. https://doi.org/10.1375/twin.9.4.523.
Manning K, Garey L, Paulus DJ, Buckner JD, Hogan JBD, Schmidt NB, et al. Typology of cannabis use among adults: a latent class approach to risk and protective factors. Addict Behav. 2019;92:6–13. https://doi.org/10.1016/j.addbeh.2018.12.008.
Zhao J, Linn B, Bradizza C, Lucke J, Ruszczyk M, Stasiewicz P. Heterogeneity in DSM-5 symptom criteria: phenotypes of alcohol use disorder in a sample seeking alcohol treatment. Alcohol Alcohol. 2021;56:660–8. https://doi.org/10.1093/alcalc/agaa138.
Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict. 1992;87:1415–31. https://doi.org/10.1111/j.1360-0443.1992.tb01921.x.
Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. https://doi.org/10.1001/archpsyc.1992.01820080007002.
Ball SA, Carroll KM, Babor TF, Rounsaville BJ. Subtypes of cocaine abusers: support for a type A-type B distinction. J Consult Clin Psychol. 1995;63:115–24. https://doi.org/10.1037//0022-006x.63.1.115.
Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–8. https://doi.org/10.1001/archpsyc.1981.01780330019001.
Epstein EE, Labouvie E, McCrady BS, Jensen NK, Hayaki J. A multi-site study of alcohol subtypes: classification and overlap of unidimensional and multi-dimensional typologies. Addiction. 2002;97:1041–53. https://doi.org/10.1046/j.1360-0443.2002.00164.x.
Litt MD, Babor TF, DelBoca FK, Kadden RM, Cooney NL. Types of alcoholics, II. Application of an empirically derived typology to treatment matching. Arch Gen Psychiatry. 1992;49:609–14. https://doi.org/10.1001/archpsyc.1992.01820080017003.
Minhas M, Oshri A, Amlung M, Dennhardt A, Ferro M, Halladay J, et al. Latent profile analysis of heavy episodic drinking in emerging adults: a reinforcer pathology approach. Alcohol Clin Exp Res. 2020;44:2130–40. https://doi.org/10.1111/acer.14438.
Syan SK, Minhas M, Oshri A, Costello J, Sousa S, Samokhvalov AV, et al. Predictors of premature treatment termination in a large residential addiction medicine program. J Subst Abus Treat. 2020;117:108077. https://doi.org/10.1016/j.jsat.2020.108077.
Tam TW, Mulia N, Schmidt LA. Applicability of type A/B alcohol dependence in the general population. Drug Alcohol Depend. 2014;138:169–76. https://doi.org/10.1016/j.drugalcdep.2014.02.698.
Wittchen HU, Behrendt S, Höfler M, Perkonigg A, Rehm J, Lieb R, et al. A typology of cannabis-related problems among individuals with repeated illegal drug use in the first three decades of life: evidence for heterogeneity and different treatment needs. Drug Alcohol Depend. 2009;102:151–7. https://doi.org/10.1016/j.drugalcdep.2009.02.012.
Albein-Urios N, Pilatti A, Lozano Ó, Martínez-González JM, Verdejo-García A. The value of impulsivity to define subgroups of addicted individuals differing in personality dysfunction, craving, psychosocial adjustment, and wellbeing: a latent class analysis. Arch Clin Neuropsychol. 2014;29:38–46. https://doi.org/10.1093/arclin/act072.
García-Marchena N, Ladrón de Guevara-Miranda D, Pedraz M, Araos PF, Rubio G, Ruiz JJ, et al. Higher impulsivity as a distinctive trait of severe cocaine addiction among individuals treated for cocaine or alcohol use disorders. Front Psychiatry. 2018;9:26.
Witkiewitz K, Roos CR, Mann K, Kranzler HR. Advancing precision medicine for alcohol use disorder: replication and extension of reward drinking as a predictor of naltrexone response. Alcohol Clin Exp Res. 2019;43:2395–405. https://doi.org/10.1111/acer.14183.
Glöckner-Rist A, Lémenager T, Mann K. Reward and relief craving tendencies in patients with alcohol use disorders: results from the PREDICT study. Addict Behav. 2013;38:1532–40. https://doi.org/10.1016/j.addbeh.2012.06.018.
Nooner K, Colcombe S, Tobe R, Mennes M, Benedict M, Moreno A, et al. The NKI-Rockland Sample: a model for accelerating the pace of discovery science in psychiatry. Front Neurosci. 2012;6:152. https://doi.org/10.3389/fnins.2012.00152.
Van Dam NT, O’Connor D, Marcelle ET, Ho EJ, Craddock RC, Tobe RH, et al. Data-driven phenotypic categorization for neurobiological analyses: beyond DSM-5 labels. Biol Psychiatry. 2017;81:484–94. https://doi.org/10.1016/j.biopsych.2016.06.027.
Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107:263–75. https://doi.org/10.1111/j.1360-0443.2011.03657.x.
Kyriazos TA. Applied psychometrics: sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. Psychology. 2018;09:2207–30. https://doi.org/10.4236/psych.2018.98126.
Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–85. https://doi.org/10.1007/BF02289447.
Glorfeld LW. An improvement on Horn’s parallel analysis methodology for selecting the correct number of factors to retain. Educ Psychol Meas. 1995;55:377–93. https://doi.org/10.1177/0013164495055003002.
Revelle WR. psych: Procedures for personality and psychological research. 2017. https://www.scholars.northwestern.edu/en/publications/psych-procedures-for-personality-and-psychological-research. Accessed 6 Jul 2021.
Huckins JF, Adeyemo B, Miezin FM, Power JD, Gordon EM, Laumann TO, et al. Reward-related regions form a preferentially coupled system at rest. Hum Brain Mapp. 2019;40:361–76. https://doi.org/10.1002/hbm.24377.
Oberski D. Mixture models: latent profile and latent class analysis. In: Robertson J, Kaptein M, editors. Modern statistical methods for HCI. Human–computer interaction series. New York City, NY: Springer International Publishing; 2016. pp. 275–87.
Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97:611–31. https://doi.org/10.1198/016214502760047131.
Scrucca L, Fop M, Murphy TB, Raftery AE. mclust 5: Clustering, classification and density estimation using gaussian finite mixture models. R J. 2016;8:289–317.
Dasgupta A, Raftery AE. Detecting features in spatial point processes with clutter via model-based clustering. J Am Stat Assoc. 1998;93:294–302. https://doi.org/10.1080/01621459.1998.10474110.
Fraley C, Raftery AE. How many clusters? Which clustering method? Answers via model-based cluster analysis. Comput J. 1998;41:578–88. https://doi.org/10.1093/comjnl/41.8.578.
Tein JY, Coxe S, Cham H. Statistical power to detect the correct number of classes in latent profile analysis. Struct Equ Model Multidiscip J. 2013;20:640–57. https://doi.org/10.1080/10705511.2013.824781.
Ferguson SL G, Moore EW, Hull DM. Finding latent groups in observed data: a primer on latent profile analysis in Mplus for applied researchers. Int J Behav Dev. 2020;44:458–68. https://doi.org/10.1177/0165025419881721.
Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo Simulation Study. Struct Equ Model Multidiscip J. 2007;14:535–69. https://doi.org/10.1080/10705510701575396.
Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F. E1071: Misc functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. 2021. https://CRAN.R-project.org/package=e1071. Accessed 6 Jul 2021.
Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS ONE. 2017;12:e0184661 https://doi.org/10.1371/journal.pone.0184661.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6. https://doi.org/10.1038/s41592-018-0235-4.
Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. https://doi.org/10.1016/j.neuroimage.2009.06.060.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. https://doi.org/10.1006/nimg.2002.1132.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042.
Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. https://doi.org/10.1016/j.neuroimage.2012.08.052.
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41. https://doi.org/10.1016/j.neuroimage.2013.08.048.
Yang L, Wu B, Fan L, Huang S, Vigotsky AD, Baliki MN, et al. Dissimilarity of functional connectivity uncovers the influence of participant’s motion in functional magnetic resonance imaging studies. Hum Brain Mapp. 2021;42:713–23. https://doi.org/10.1002/hbm.25255.
Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–8. https://doi.org/10.1038/nature18933.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–24. https://doi.org/10.1016/j.neuroimage.2013.04.127.
Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. https://doi.org/10.1146/annurev-clinpsy-040510-143934.
Garrison KA, Scheinost D, Finn ES, Shen X, Constable RT. The (in)stability of functional brain network measures across thresholds. Neuroimage. 2015;118:651–61. https://doi.org/10.1016/j.neuroimage.2015.05.046.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. https://doi.org/10.1016/j.neuroimage.2009.10.003.
Achard S. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. https://doi.org/10.1523/JNEUROSCI.3874-05.2006.
Oldham S, Fulcher B, Parkes L, Arnatkevic̆iūtė A, Suo C, Fornito A. Consistency and differences between centrality measures across distinct classes of networks. PLoS ONE. 2019;14:e0220061. https://doi.org/10.1371/journal.pone.0220061.
Marcus D, Harwell J, Olsen T, Hodge M, Glasser M, Prior F, et al. Informatics and data mining tools and strategies for the Human Connectome Project. Front Neuroinformatics. 2011;5:4. https://doi.org/10.3389/fninf.2011.00004.
Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–37. https://doi.org/10.1162/jocn_a_00077.
Watkins MW. Exploratory factor analysis: a guide to best practice. J Black Psychol. 2018;44:219–46. https://doi.org/10.1177/0095798418771807.
Goretzko D, Pham TTH, Bühner M. Exploratory factor analysis: current use, methodological developments and recommendations for good practice. Curr Psychol. 2021;40:3510–21. https://doi.org/10.1007/s12144-019-00300-2.
Carlyle M, Broomby R, Simpson G, Hannon R, Fawaz L, Mollaahmetoglu OM, et al. A randomised, double-blind study investigating the relationship between early childhood trauma and the rewarding effects of morphine. Addict Biol. 2021;26:e13047. https://doi.org/10.1111/adb.13047.
Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Cogn Brain Res. 2005;25:851–61. https://doi.org/10.1016/j.cogbrainres.2005.09.018.
Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, Robbins TW. Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biol Psychiatry. 2013;74:137–44. https://doi.org/10.1016/j.biopsych.2012.11.016.
Gray JC, MacKillop J. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychol Addict Behav. 2014;28:282–7. https://doi.org/10.1037/a0032766.
Jonker NC, Ostafin BD, Glashouwer KA, van Hemel-Ruiter ME, de Jong PJ. Reward and punishment sensitivity and alcohol use: the moderating role of executive control. Addict Behav. 2014;39:945–8. https://doi.org/10.1016/j.addbeh.2013.12.011.
Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. https://doi.org/10.1007/s00213-006-0487-z.
Schlauch RC, Christensen RL, Derrick JL, Crane CA, Collins RL. Individual differences in approach and avoidance inclinations moderate the effect of self-control depletion on ad-lib drinking. Alcohol Clin Exp Res. 2015;39:2480–8. https://doi.org/10.1111/acer.12915.
Stevens AK, Littlefield AK, Talley AE, Brown JL. Do individuals higher in impulsivity drink more impulsively? A pilot study within a high risk sample of young adults. Addict Behav. 2017;65:147–53. https://doi.org/10.1016/j.addbeh.2016.10.026.
White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2006;31:1064–74. https://doi.org/10.1038/sj.npp.1300939.
Belcher AM, Volkow ND, Moeller FG, Ferré S. Personality traits and vulnerability or resilience to substance use disorders. Trends Cogn Sci. 2014;18:211–7. https://doi.org/10.1016/j.tics.2014.01.010.
Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. https://doi.org/10.1186/1741-7015-11-126.
Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: the NIMH research domain criteria. J Abnorm Psychol. 2013;122:928–37. https://doi.org/10.1037/a0034028.
Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Bowden-Jones H, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114:1095. https://doi.org/10.1111/add.14424.
Zilverstand A, Goldstein RZ. Chapter 3 - Dual models of drug addiction: the impaired response inhibition and salience attribution model. In: Verdejo-Garcia A, editor. Cognition and addiction. Cambridge, MA: Academic Press; 2020. pp. 17–23.
Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80:179–89. https://doi.org/10.1016/j.biopsych.2015.10.024.
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–7. https://doi.org/10.1162/089892902317361886.
Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan executive function system: an update. J Int Neuropsychol Soc. 2004;10:301–3. https://doi.org/10.1017/S1355617704102191.
Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–62. https://doi.org/10.1016/j.jneumeth.2009.11.017.
Rawls E, Kummerfeld E, Zilverstand A. An integrated multimodal model of alcohol use disorder generated by data-driven causal discovery analysis. Commun Biol. 2021;4:1–12. https://doi.org/10.1038/s42003-021-01955-z.
Naragon-Gainey K, Gallagher MW, Brown TA. Stable “trait” variance of temperament as a predictor of the temporal course of depression and social phobia. J Abnorm Psychol. 2013;122:611–23. https://doi.org/10.1037/a0032997.
Prenoveau JM, Craske MG, Zinbarg RE, Mineka S, Rose RD, Griffith JW. Are anxiety and depression just as stable as personality during late adolescence? Results from a three-year longitudinal latent variable study. J Abnorm Psychol. 2011;120:832–43. https://doi.org/10.1037/a0023939.
Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137:201–25. https://doi.org/10.1037/0096-3445.137.2.201.
Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39. https://doi.org/10.1016/j.neubiorev.2012.01.002.
Chase HW, Kumar P, Eickhoff SB, Dombrovski AY. Reinforcement learning models and their neural correlates: an activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci. 2015;15:435–59. https://doi.org/10.3758/s13415-015-0338-7.
Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–8. https://doi.org/10.1126/science.1142995.
Schoenbaum G, Chang CY, Lucantonio F, Takahashi YK. Thinking outside the box: orbitofrontal cortex, imagination, and how we can treat addiction. Neuropsychopharmacology. 2016;41:2966–76. https://doi.org/10.1038/npp.2016.147.
Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. https://doi.org/10.1016/j.neuron.2008.04.017.
Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40. https://doi.org/10.1016/j.neuron.2013.07.007.
Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–95. https://doi.org/10.1016/j.neuroimage.2012.01.117.
Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. https://doi.org/10.1016/j.neuron.2018.03.048.
Bi XA, Sun Q, Zhao J, Xu Q, Wang L. Non-linear ICA analysis of resting-state fMRI in mild cognitive impairment. Front Neurosci. 2018;12:413. https://doi.org/10.3389/fnins.2018.00413.
Zhang Y, Zhang S, Ide JS, Hu S, Zhornitsky S, Wang W, et al. Dynamic network dysfunction in cocaine dependence: graph theoretical metrics and stop signal reaction time. Neuroimage Clin. 2018;18:793–801. https://doi.org/10.1016/j.nicl.2018.03.016.
Zilverstand A, O’Halloran R, Goldstein RZ. Resting-state and structural brain connectivity in individuals with stimulant addiction: a systematic review. In: The Routledge handbook of philosophy and science of addiction. Oxfordshire: Routledge; 2018.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85. https://doi.org/10.1016/j.tics.2013.12.003.
Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. https://doi.org/10.1016/j.tics.2011.11.009.
Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. a systematic review. Neuroimage. 2017;151:105–16. https://doi.org/10.1016/j.neuroimage.2016.06.009.
Chen NTM, Thomas LM, Clarke PJF, Hickie IB, Guastella AJ. Hyperscanning and avoidance in social anxiety disorder: the visual scanpath during public speaking. Psychiatry Res. 2015;225:667–72. https://doi.org/10.1016/j.psychres.2014.11.025.
Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: a critical review. Cogn Ther Res. 2009;33:221–34. https://doi.org/10.1007/s10608-007-9161-y.
Mann K, Hermann D. Individualised treatment in alcohol-dependent patients. Eur Arch Psychiatry Clin Neurosci. 2010;260:116–20. https://doi.org/10.1007/s00406-010-0153-7.
Babor TF, Caetano R. Subtypes of substance dependence and abuse: implications for diagnostic classification and empirical research. Addiction. 2006;101(Suppl 1):104–10. https://doi.org/10.1111/j.1360-0443.2006.01595.x.
Connor JP, Gullo MJ, White A, Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. 2014;27:269–75. https://doi.org/10.1097/YCO.0000000000000069.
Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39:579–84. https://doi.org/10.1111/acer.12669.
Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5:eaax4043. https://doi.org/10.1126/sciadv.aax4043.
Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, et al. Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology. 2018;43:891–9. https://doi.org/10.1038/npp.2017.282.
Hobbs JDJ, Kushner MG, Lee SS, Reardon SM, Maurer EW. Meta-analysis of supplemental treatment for depressive and anxiety disorders in patients being treated for alcohol dependence. Am J Addict. 2011;20:319–29. https://doi.org/10.1111/j.1521-0391.2011.00140.x.
Kushner MG, Maurer EW, Thuras P, Donahue C, Frye B, Menary KR, et al. Hybrid cognitive behavioral therapy versus relaxation training for co-occurring anxiety and alcohol disorder: a randomized clinical trial. J Consult Clin Psychol. 2013;81:429–42. https://doi.org/10.1037/a0031301.
Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive function as a transdiagnostic treatment target in stimulant use disorders. J Dual Diagn. 2016;12:90–106. https://doi.org/10.1080/15504263.2016.1146383.
Bickel WK, Moody L, Quisenberry A. Computerized working-memory training as a candidate adjunctive treatment for addiction. Alcohol Res Curr Rev. 2014;36:123–6.
Song S, Zilverstand A, Gui W, Pan X, Zhou X. Reducing craving and consumption in individuals with drug addiction, obesity or overeating through neuromodulation intervention: a systematic review and meta-analysis of its follow-up effects. Addiction. 2022;117:1242–55. https://doi.org/10.1111/add.15686.
Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12:606–18. https://doi.org/10.1016/j.brs.2018.12.975.
Bickel WK, Athamneh LN, Snider SE, Craft WH, DeHart WB, Kaplan BA, et al. Reinforcer pathology: implications for substance abuse intervention. Curr Top Behav Neurosci. 2020;47:139–62. https://doi.org/10.1007/7854_2020_145.
DiClemente CC, Corno CM, Graydon MM, Wiprovnick AE, Knoblach DJ. Motivational interviewing, enhancement, and brief interventions over the last decade: a review of reviews of efficacy and effectiveness. Psychol Addict Behav. 2017;31:862–87. https://doi.org/10.1037/adb0000318.
Domínguez-Salas S, Díaz-Batanero C, Lozano-Rojas OM, Verdejo-García A. Impact of general cognition and executive function deficits on addiction treatment outcomes: systematic review and discussion of neurocognitive pathways. Neurosci Biobehav Rev. 2016;71:772–801. https://doi.org/10.1016/j.neubiorev.2016.09.030.
Foulds J, Newton-Howes G, Guy NH, Boden JM, Mulder RT. Dimensional personality traits and alcohol treatment outcome: a systematic review and meta-analysis. Addiction. 2017;112:1345–57. https://doi.org/10.1111/add.13810.
Moeller SJ, Bederson L, Alia-Klein N, Goldstein RZ. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog Brain Res. 2016;223:165–88. https://doi.org/10.1016/bs.pbr.2015.07.007.
Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abus Treat. 2014;47:58–72. https://doi.org/10.1016/j.jsat.2014.01.008.
Acknowledgements
We would like to thank Dr. Kathryn R. Cullen, Andrea M. Maxwell, Dr. Sarah R. Heilbronner, and Dr. Jan Zimmermann for their helpful comments regarding the manuscript.
Funding
This work was supported by grants from the National Institute on Drug Abuse (5T32DA007234-29 to GD) and from the National Institute of Mental Health (T32-MH115866 to ER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
GD: data curation, validation, formal analysis, writing—original draft, review, and editing, visualization. LB: conceptualization, methodology, software, data curation, validation, formal analysis, software, writing—review and editing, visualization. ER: methodology, resources, writing—review and editing. TH: methodology, software, resources, writing—review and editing. AZ: conceptualization, methodology, resources, writing—original draft, review, and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Drossel, G., Brucar, L.R., Rawls, E. et al. Subtypes in addiction and their neurobehavioral profiles across three functional domains. Transl Psychiatry 13, 127 (2023). https://doi.org/10.1038/s41398-023-02426-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02426-1
This article is cited by
-
Incubation of methamphetamine craving in punishment-resistant individuals is associated with activation of specific gene networks in the rat dorsal striatum
Molecular Psychiatry (2024)
-
Towards personalized medicine: subtyping using functional profiles
Neuropsychopharmacology (2024)
-
Data-driven connectivity profiles relate to smoking cessation outcomes
Neuropsychopharmacology (2024)
-
A Systematic Review of Sex/Gender Differences in the Multi-dimensional Neurobiological Mechanisms in Addiction and Their Relevance to Impulsivity
Current Addiction Reports (2023)