Abstract
Dysregulated sleep is commonly reported in individuals with neuropsychiatric disorders, including schizophrenia (SCZ) and bipolar disorder (BPD). Physiology and pathogenesis of these disorders points to aberrant metabolism, during neurodevelopment and adulthood, of tryptophan via the kynurenine pathway (KP). Kynurenic acid (KYNA), a neuroactive KP metabolite derived from its precursor kynurenine by kynurenine aminotransferase II (KAT II), is increased in the brains of individuals with SCZ and BPD. We hypothesize that elevated KYNA, an inhibitor of glutamatergic and cholinergic neurotransmission, contributes to sleep dysfunction. Employing the embryonic kynurenine (EKyn) paradigm to elevate fetal brain KYNA, we presently examined pharmacological inhibition of KAT II to reduce KYNA in adulthood to improve sleep quality. Pregnant Wistar rats were fed either kynurenine (100 mg/day)(EKyn) or control (ECon) diet from embryonic day (ED) 15 to ED 22. Adult male (N = 24) and female (N = 23) offspring were implanted with devices to record electroencephalogram (EEG) and electromyogram (EMG) telemetrically for sleep-wake data acquisition. Each subject was treated with either vehicle or PF-04859989 (30 mg/kg, s.c.), an irreversible KAT II inhibitor, at zeitgeber time (ZT) 0 or ZT 12. KAT II inhibitor improved sleep architecture maintaining entrainment of the light-dark cycle; ZT 0 treatment with PF-04859989 induced transient improvements in rapid eye movement (REM) and non-REM (NREM) sleep during the immediate light phase, while the impact of ZT 12 treatment was delayed until the subsequent light phase. PF-04859989 administration at ZT 0 enhanced NREM delta spectral power and reduced activity and body temperature. In conclusion, reducing de novo KYNA production alleviated sleep disturbances and increased sleep quality in EKyn, while also improving sleep outcomes in ECon offspring. Our findings place attention on KAT II inhibition as a novel mechanistic approach to treating disrupted sleep behavior with potential translational implications for patients with neurodevelopmental and neuropsychiatric disorders.
Similar content being viewed by others
Introduction
More than one third of the global population currently reports problems with sleep [1]. In patients with severe psychiatric disorders, the risk of irregular sleep patterns is two-fold higher compared to healthy controls, and thus, expectedly, the prevalence of sleep disturbances is 80% amongst individuals with schizophrenia (SCZ) and bipolar disorder (BPD)[2, 3]. The causation and relationship between psychiatric disorders and sleep is complex and bi-directional [4, 5]. As sleep is a universal, physiological process that serves a restorative function to the body, the loss and disruption of sleep in patients with psychiatric illness often exacerbates their symptom severity. For decades, besides benzodiazepines and melatonin [6, 7], clinical therapies for sleep disorders are associated with increased incidence of adverse effects such as sedation, daytime somnolence, and cognitive decline [8, 9]. Therefore, optimizing novel therapies for clinical treatment of sleep disorders related to neuropsychiatric conditions has emerged as a high priority to improve health outcomes for patients.
Metabolites of the kynurenine pathway (KP) play a major role in both physiology and pathogenesis of major psychiatric disorders [10, 11]. More than 95% of the ingested essential amino acid tryptophan is catabolized by the KP to generate nicotinamide adenine dinucleotide (NAD+), the ubiquitous co-factor and crucial energy source, as well as several neuroactive metabolites [12, 13]. Elevated levels of kynurenic acid (KYNA), a kynurenine-derived gliotransmitter that inhibits glutamatergic and cholinergic neurotransmission [14,15,16], have been found in the postmortem brain and cerebrospinal fluid of individuals with SCZ and BPD [17,18,19,20,21]. Inhibition of kynurenine aminotransferase (KAT) II, an enzyme which accounts for ~75% of the brain KYNA neosynthesis [22], elicits reduction of KYNA in the brain [23,24,25], which could have a direct impact on brain function and neurobiological processes relevant to SCZ and BPD.
Elevations in KYNA have also been implicated in pathogenic mechanisms that contribute to aberrant neurodevelopment which precedes the clinical onset of SCZ and BPD [26,27,28]. Embryonic kynurenine (EKyn) experimental paradigm was established as a neurodevelopmental model that simulated in utero upregulation of the KP [27, 29]. EKyn pregnant rats are treated with kynurenine during the last week of rodent gestation, translating to the second trimester in human pregnancy [30, 31], to induce a prenatal increase in KYNA. Notably, young adult EKyn offspring demonstrate sex-specific and circadian-dependent changes in KP metabolism in the hippocampus and the prefrontal cortex (PFC) [29, 32,33,34], namely increased KYNA within the brain of male offspring [32, 35]. Stark cognitive behavioral impairments have been reported across studies in EKyn offspring [29, 33, 36, 37], which may be related to notable disruptions in sleep architecture, including shorter rapid eye movement (REM) duration in males, and prolonged episodes of quiescent wake behavior in females [32, 38].
Our present goal was to pharmacologically inhibit KAT II in an effort to reduce brain KYNA synthesis and to promote healthier sleep dynamics in embryonic control (ECon) and EKyn rats of both sexes. Subjects were treated acutely with PF-04859989 [25], a brain-penetrable and irreversible KAT II inhibitor, at the start of the largely quiescent light phase or more active dark phase, to evaluate implications of reducing KYNA with a chronopharmacological approach [39]. We determined that this compound enhanced REM and non-REM (NREM) sleep, and importantly, KAT II inhibition in EKyn offspring restored sleep physiology to the level of counterpart controls. Together, these findings place novel attention on the translational value of KAT II inhibition for treating sleep deficiencies and improving sleep architecture.
Methods
Animals
Pregnant Wistar rats, embryonic day (ED) 2, were obtained from Charles River Laboratories (Raleigh, NC, USA). All experimental animals were housed in a temperature- and humidity-controlled facility, fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at the University of South Carolina School of Medicine. The rats had free access to food and water and were maintained on a 12 h/12 h light-dark cycle, where lights on corresponded to zeitgeber time (ZT) 0 and lights off to ZT 12. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Embryonic kynurenine (EKyn) treatment
Rodent chow was finely ground in a blender, and each dam received approximately 30 grams of wet rodent mash daily (ECon). Embryonic kynurenine (EKyn) dams were fed 30 grams of rodent mash daily with 100 mg/day of L-kynurenine sulfate (purity 99.4%, Sai Advantium, Hyderabad, India), from ED 15 through ED 22 as previously described [29]. From the day of birth, postnatal day (PD) 0, standard rodent chow pellets were provided to all animals ad libitum. On PD 21, male and female offspring were weaned and pair-housed by litter and sex. Offspring were used experimentally from PD 56 to PD 85 (experimental timeline in Fig. 1). To minimize the contribution of individual prenatal litters, the distribution of progeny from any given litter was one to two pups per sex for all experiments, as deemed appropriate by our previously published studies [37, 38, 40]. For sleep experiments, a total of 24 male (12 ECon; 12 EKyn) and 23 female (11 ECon; 12 EKyn) offspring were used. For behavioral experiments, a total of 48 male (25 ECon; 23 EKyn) and 54 female (26 ECon; 28 EKyn) offspring were used. Experimenters were blind to group during experiments in adulthood.
Embryonic kynurenine (EKyn) treatment: from embryonic day (ED) 15 through ED 22 dams were fed daily with control diet (ECon) or diet laced with 100 mg kynurenine (EKyn). Sleep studies were performed in adult offspring between PD 56–85. In experiment 1, each subject was treated with vehicle or PF-04859989 (30 mg/kg) at the beginning of light phase, Zeitgeber time (ZT) 0. In experiment 2, each subject was treated with vehicle or PF-04859989 (30 mg/kg) at the beginning of dark phase, ZT 12. Electroencephalogram (EEG), electromyograph (EMG), and cage activity data were inspected in 10-s epochs and classified into one of three states: rapid eye movement (REM), non-REM (NREM), and wake. Artificial neural network (ANN) was implemented for highly accurate predictive classification of vigilance states.
Surgery
Surgical implantation of telemetry transmitters (HD-S02; Data Sciences International (DSI), St. Paul, MN, USA) was according to the previously published protocols [38, 41]. Briefly, animals were anesthetized using isoflurane anesthesia (induction 5%; maintenance 1.5–2.5%) and placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA) to secure the head. At the beginning of the surgical procedure, carprofen (5 mg/kg, s.c.) was administered as an analgesic. The telemetry transmitter was implanted intraperitoneally through a longitudinal incision made on the dorsal abdominal surface. Along the midline of the head and neck, another longitudinal incision was made to expose the skull and neck muscle, and electroencephalograph (EEG) and electromyograph (EMG) electrode leads were threaded subcutaneously to the incision. Two burr holes (0.5 mm diameter) were drilled and two surgical stainless-steel screws (P1 Technologies, Roanoke, VA, USA) were implanted at 2.0 mm anterior/ +1.5 mm lateral and 7.0 mm posterior/−1.5 mm lateral relative to bregma. The two EEG leads were wrapped around the screws and secured with dental cement (Stoelting Co., Wood Dale, IL, USA). The two EMG leads were inserted directly into the dorsal cervical neck muscle approximately 1.0 mm apart and sutured into place. The dorsal incision was stapled closed with wound clips, and the skin along the head was sutured. Postoperatively animals were singly housed and recovered for at least 7 days prior to the start of experiments and remained singly housed for the duration of studies.
Sleep data acquisition and analysis
Sleep behavior was evaluated in a within animal design. Each subject received injections at ZT 0 for experiment 1 (male: ECon N = 8, EKyn N = 8; female: ECon N = 7, EKyn N = 8) and at ZT 12 for experiment 2 (sexes combined: ECon N = 8, EKyn N = 8). For both experiments, each animal was treated with vehicle (ultrapure water) on the first data acquisition day and PF-04859989 (purity 95%, WuXi AppTec, Shanghai, China)(30 mg/kg, s.c.) during the other acquisition day (Fig. 1). In an effort to match estrous phase in female subjects and allow ample time for drug wash out, treatments were spaced 3–4 days apart. Sleep data were collected for 24 h after each injection.
All sleep data were recorded in a designated room where animals were singly housed, and cages were placed on receivers that relayed data continuously to Ponemah 6.10 (DSI). Digitized signal data were processed offline with NeuroScore 3.0 (DSI). According to visual inspection of EEG/EMG waveforms and confirmation with a trained artificial neural network (ANN)(to 95% agreement; details below), 10-s epochs were classified into one of three states: wake, NREM, or REM. Wake was characterized by low-amplitude, high-frequency EEG combined with high-amplitude EMG; NREM by high-amplitude, low-frequency EEG combined with low-amplitude EMG; and REM by low-amplitude, high-frequency EEG combined with very low EMG tone and muscle atonia (see Fig. 1). An uninterrupted episode of a single vigilance state lasting for at least one full epoch was defined as a bout, and a transition was scored when two or more epochs were noted as the new vigilance state.
The scored data for each vigilance state were analyzed in 1-h time bins and 6-h time bins for total duration, number of bouts, average bout duration, relative cage activity, and core body temperature. NREM and REM sleep onset and the number of transitions between vigilance states were determined for each 12-h cycle. Power spectrum analysis during REM and NREM sleep, separated by phase, was evaluated with Discrete Fourier Transformation (DFT) to obtain the power spectrum for the following frequency bandwidths: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–16 Hz), and beta (16–20 Hz).
Artificial neural network (ANN)
We have shown that an ANN, a popular supervised predictive machine learning technique, is highly accurate in classifying vigilance state (wake, NREM, or REM) in rats based on features extracted from their polysomnography [42]. We trained and verified the correctness of the ANN by using over 400 h of continuously monitored EEG and EMG signals, along with relative home cage activity. Signals were partitioned into 10-s epochs where each epoch was then individually transformed to the frequency domain using DFT. Power spectrum and total power were computed for 40 channels of equal width from 0 to 20 Hz. Along with average EMG and relative home cage activity, 42 features result for each epoch. A simple moving window of five epochs was used to create input samples consisting of 210 features (42 features for each epoch in the window), and labels were assigned based on the most common class among the five epochs. To prevent overfitting and ensure generalization, the data was partitioned into training (64%), validation (16%), and testing (20%) sets. We observed an imbalanced class distribution such that the number of samples assigned to each vigilance state was not uniformly distributed. To mitigate this issue, we used a resampling technique, oversampling, to balance the class distribution in the training data. The use of resampling techniques allowed us to address the issue of imbalanced classes and ensure that our ANN was trained on a balanced and representative dataset [42]. The ANN used in our study is fully connected, consisting of 210 input neurons, 512 hidden neurons with a Rectified Linear Unit (RELU) activation function, and 3 output neurons with a Softmax activation function. The network was calibrated on the training and validation data until validation loss plateaued. Evaluation of the ANN was performed on the testing data using metrics such as accuracy, F1 score, and a confusion matrix. Our results indicate a high degree of accuracy in the classification of vigilance states, which was further verified by a domain expert through visual inspection of the model’s predictions.
Open field test
Open field test was used to examine exploratory behavior and spontaneous locomotor activity in rats. Experiments were conducted during the light phase (ZT 2–4) with lights on (male: ECon N = 14, EKyn N = 12; female: ECon N = 12, EKyn N = 15) or during the dark phase (ZT 22–24) under red light (male: ECon N = 11, EKyn N = 11; female ECon N = 14, EKyn N = 13). An open field arena (70 cm × 70 cm) was divided into a square central zone in the middle (35 cm × 35 cm), a peripheral zone which consisted of four corners (17.5 cm × 17.5 cm), and a perimeter zone (17.5 cm from arena walls). Each rat was placed in the center of the open field arena and allowed to freely explore for 30 min. The animal’s movement was tracked with an overhead video system using EthoVision XT 15 software (Noldus, Leesburg, VA, USA). Total distance traveled, velocity, time spent in the center of the arena, and time spent in the corners of the arena were assessed.
Statistical analysis
Distribution of data was assessed with Shapiro–Wilk test and visual inspection of the data using Q–Q plots to confirm a relative bell-shaped distribution and an absence of outliers. Initially, sleep architecture data were compared by a three-way repeated-measures (RM) analysis of variance (ANOVA) as follows: total duration of vigilance states (REM, NREM, wake) in 1-h time bins with treatment (vehicle or PF-04859989) and the time of day (ZT) as within-subject factors and sex (male or female) as a between-subject factor; core body temperature and relative cage activity in 1-h time bins with treatment (vehicle or PF-04859989) and the time of day (ZT) as within-subject factors and prenatal condition (ECon or EKyn) as a between-subject factor; and NREM and REM onset and transitions between vigilance states with treatment (vehicle or PF-04859989) and phase (light or dark) as within-subject factors and prenatal condition (ECon or EKyn) as a between-subject factor. Significant main effect of treatment and interactions with treatment were analyzed by two-way RM ANOVA as follows: total duration of vigilance states (REM, NREM, wake), number of bouts, and average bout duration in 6-h time bins with the time of day (ZT) as a within-subject factor; EEG power spectra, NREM delta power and REM theta power, with frequency as a within-subject factor; absolute change in total duration of vigilance states (REM, NREM, wake) and NREM delta spectral power with the time of day (ZT) and frequency as a within-subject factor, respectively; and NREM and REM onset with the prenatal condition (ECon and EKyn) as a between-subject factor. Relative cage activity and core body temperature were analyzed by mixed-effects analysis for each prenatal condition separately. Impact of PF-04859989 treatment on vigilance state duration and relative cage activity was evaluated by calculating the percent change from vehicle. The absolute difference in vigilance state duration and NREM delta spectral power were calculated as a within-subject difference between treatments. Open field data were evaluated by a two-way ANOVA with prenatal condition (ECon or EKyn) and phase (light or dark) as a between-subject factors. In all analyses, where appropriate, significant main effects were followed up with the Fisher’s LSD post hoc analyses. Statistical significance was defined as P < 0.05. All statistical analyses were performed using GraphPad Prism 9.0 software (GraphPad Software, La Jolla, CA, USA). Statistical comparisons and effects sizes are provided in the figure legends.
Results
To evaluate the impact of reducing KYNA formation on sleep and arousal in adult rats, sleep parameters were assessed in young adult male and female ECon and EKyn offspring after PF-04859989 treatment at ZT 0 or ZT 12. The experimental dose of PF-04859989 was selected based on previously published reports wherein extracellular KYNA levels were substantially reduced across brain regions [25, 43].
Early light phase KAT II inhibition enhances sleep
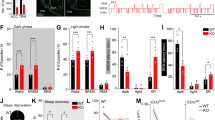
KYNA levels are elevated during the light phase in EKyn offspring, which may causally contribute to decreased REM duration and number of REM bouts [32, 35, 38]. Thus, we presently sought to determine if inhibition of KYNA synthesis via systemic treatment with a KAT II inhibitor during the light phase would restore REM sleep parameters. When treated with PF-04859989 at ZT 0, REM duration was significantly enhanced across 24 h in both ECon and EKyn offspring, and analyses separated by sex revealed a significant effect of PF-04859989 in male rats (Fig. 2A, B). Total REM duration was increased by 18–19% during the light phase in ECon, and by 31% during the second half of the light phase in EKyn offspring (Fig. 2C, D), suggesting a rapid enhancement in REM sleep following PF-04859989 treatment.
Adult ECon and EKyn offspring were treated with vehicle or PF-04859989 (30 mg/kg) at Zeitgeber time (ZT) 0. A 1-h bins of REM duration in male and female ECon (three-way ANOVA: treatment effect F(1, 13) = 10.00, P < 0.01; Two-way ANOVA: Male Treatment effect F(1, 7) = 8.938, P < 0.05). B 1-h bins of REM duration in male and female EKyn (three-way ANOVA: Treatment effect F(1, 14) = 11.76, P < 0.01; two-way ANOVA: male treatment effect F(1, 7) = 7.211, P < 0.05). C 6-h bins of REM duration in ECon, sexes combined (Treatment effect F(1, 14) = 10.22, P < 0.01). D 6-h bins of REM duration in EKyn, sexes combined (Treatment effect F(1, 15) = 11.15, P < 0.01). E 1-h bins of NREM duration in male and female ECon (Three-way ANOVA: treatment effect F(1, 13) = 6.135, P < 0.05, treatment × sex × ZT interaction F(23, 281) = 2.005, P < 0.01; two-way ANOVA: male treatment effect F(1, 7) = 6.976, P < 0.05, female treatment × ZT interaction F(23, 120) = 2.088, P < 0.01). F 1-h bins of NREM duration in male and female EKyn (three-way ANOVA: treatment effect F(1, 14)= 8.530, P < 0.05, treatment × ZT interaction F(23, 310) = 1.850, P < 0.05, treatment × sex × ZT interaction F(23, 310) = 1.780, P < 0.05; two-way ANOVA: male treatment effect F(1, 7) = 6.510, P < 0.05, female treatment × ZT interaction F(23, 149)= 2.218, P < 0.01). G 6-h bins of NREM duration in ECon, sexes combined (treatment effect F(1, 14)= 10.74, P < 0.01). H 6-h bins of NREM duration in EKyn, sexes combined (treatment effect F(1, 15) = 7.915, P < 0.05). I 1-h bins of wake duration in male and female ECon (three-way ANOVA: treatment effect F(1, 13) = 7.459, P < 0.05, treatment × ZT interaction F(23, 281) = 1.720, P < 0.05, treatment × sex × ZT interaction F(23, 281) = 2.011, P < 0.01; two-way ANOVA: male treatment effect F(1, 7) = 6.445, P < 0.05, female treatment × ZT interaction F(23, 120) = 2.114, P < 0.01). J 1-h bins of wake duration in male and female EKyn (three-way ANOVA: treatment effect F(1, 14) = 12.07, P < 0.01, treatment × ZT interaction F(23, 310) = 1.755, P < 0.05, treatment × sex × ZT interaction F(23, 310)= 1.875, P < 0.01; two-way ANOVA: male treatment effect F(1, 7) = 12.30, P < 0.01, female treatment × ZT interaction F(23, 149) = 2.235, P < 0.01). K 6-h bins of wake duration in ECon, sexes combined (treatment effect F(1, 14) = 11.98, P < 0.01). L 6-h bins of wake duration in EKyn, sexes combined (treatment effect F(1, 15) = 11.05, P < 0.01). Data are mean ± SEM. Percent change from vehicle treatment calculations are shown by arrows. Three-way RM ANOVA: #P < 0.05, ##P < 0.01. two-way RM ANOVA: ^P < 0.05, ^^P < 0.01 with Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. N = 12–16 per group.
Over 24 h, PF-04859989 treatment significantly impacted NREM duration in ECon and EKyn offspring (Figs. 2E, F). Total NREM duration was increased during the latter part of both light and dark phases in ECon, +6% and +14%, respectively, and +15% during the second half of the dark phase in EKyn offspring (Fig. 2G, H). Sleep architecture is characterized by the number and length of bouts of REM and NREM sleep. PF-04859989 treatment significantly impacted NREM bout number in ECon and EKyn rats, wherein NREM bout number increased by 17% during the late dark phase (Supplementary Fig. 1).
Each vigilance state comprises bouts of various lengths and bouts sustained for a longer time indicate a firmly consolidated vigilance state. We noted a significant treatment × ZT interaction for average duration of both REM and NREM bouts with a phase-specific effects of PF-04859989 treatment. Average REM bout duration in EKyn rats was increased by 34% during the first half and decreased by 28% during the second half of the light phase, while average NREM bout duration was increased by 13% during early light phase in ECon rats (Supplementary Fig. 1).
As REM and NREM sleep were enhanced, wakefulness was reduced. With PF-04859989 treatment at the beginning of the light phase subjects were awake less time across 24 h, determined by a main effect of treatment, a treatment × ZT interaction, as well as an interaction between treatment × sex × ZT (Fig. 2I, J). Male and female ECon and EKyn offspring exhibited reduced wake duration during the second half of light phase, −15% and −14% respectively, and dark phase, −7% and −8%, respectively (Fig. 2K, L). Taken together, treatment of adult offspring with PF-04859989 at ZT 0 induced enhanced somnolence.
KAT II inhibition enhances NREM delta spectral power
Delta power during NREM sleep correlates strongly with sleep quality [44], and theta oscillations during REM sleep are implicated in memory consolidation [45, 46]. Therefore, we next sought to detect if acutely inhibiting KAT II would be sufficient to drive changes in EEG waves within low-frequency oscillation bands, delta (0–4 Hz) and theta (4–8 Hz). When PF-04859989 was administered at the beginning of the light phase, we found significant effects of KAT II inhibition on delta spectral power during both the light and dark phases, but with subtle sex-dependent differences. In male ECon offspring, a treatment × frequency interaction pointed to a significant increase in NREM delta power immediately after the treatment during the light phase (Fig. 3A), and the effect of PF-04859989 treatment lasted through the dark phase (Fig. 3B). In adult male EKyn rats, PF-04895589 treatment significantly elevated the low-frequency delta bands only during the light phase (Fig. 3C, D). Among female offspring, NREM power in the lower frequency range was enhanced, as determined by a treatment x frequency interaction in ECon rats during the light phase alone (Fig. 3E–H). Analyses of theta spectral power after acute KYNA reduction at ZT 0 showed an interaction between treatment × frequency and alterations within REM theta power in EKyn male rats during the light phase (Supplementary Fig. 2).
Adult ECon and EKyn offspring were treated with vehicle or PF-04859989 (30 mg/kg) at Zeitgeber time (ZT) 0. A Male ECon during light phase (treatment × frequency interaction F(7, 49) = 2.892, P < 0.05). B Male ECon during dark phase (Treatment effect F(1, 7) = 6.678, P < 0.05). C Female ECon during light phase (treatment × frequency interaction F(7, 42) = 2.897, P < 0.05). D Female ECon during dark phase. E Male EKyn during light phase (Treatment effect F(1, 7) = 9.155, P < 0.05). F Male EKyn during dark phase. G Female EKyn during light phase. H Female EKyn during dark phase. Data are mean ± SEM. Two-way RM ANOVA: ^P < 0.05 with Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. N = 7–8 per group.
Dark Phase KAT II inhibition promotes sleep during the subsequent light phase
To evaluate the impact of reducing KYNA during the dark phase on vigilance state parameters, a separate cohort of subjects was administered PF-04859989 at ZT 12. Administration of KAT II inhibitor at ZT 12 elicited a treatment x ZT interaction across all three vigilance states in ECon offspring, with total REM and NREM duration increased during the first half of the successive light phase by 19% and 14%, respectively (Fig. 4A, B). Increase in sleep duration between ZT 0 and ZT 6 during the succeeding light phase coincided with 28% reduction in wake duration (Fig. 4C). During the latter part of the subsequent light phase, between ZT 6 and ZT 12, NREM duration was reduced by 11%, and wake duration increased by 21% in ECon subjects (Figs. 4B, C). Assessment of sleep architecture detected that the number of wake bouts increased with PF-04859989 treatment in ECon rats between ZT 0 and ZT 6, despite spending less time awake during this period (Supplementary Fig. 3). No changes in NREM power spectra were found with PF-04859989 treatment at ZT 12 (Fig. 4D, E).
Adult ECon and EKyn offspring were treated with vehicle or PF-04859989 (30 mg/kg) at Zeitgeber time (ZT) 12. A 6-h bins of REM duration in ECon, sexes combined (treatment × ZT interaction F(3, 21) = 3.451, P < 0.05). B 6-h bins of NREM duration in ECon, sexes combined (treatment × ZT interaction F(3, 21) = 4.355, P < 0.05). C 6-h bins of wake duration in ECon, sexes combined (treatment × ZT interaction F(3, 21) = 5.087, P < 0.01). D NREM delta spectral power during dark phase in ECon, sexes combined. E NREM delta spectral power during light phase in ECon, sexes combined. F 6-h bins of REM duration in EKyn, sexes combined. G 6-h bins of NREM duration in EKyn, sexes combined (treatment effect F(1, 7) = 5.594, P = 0.05, treatment × ZT interaction F(3, 21) = 3.290, P < 0.05). H 6-h bins of wake duration in EKyn, sexes combined (treatment effect F(1, 7) = 6.926, P < 0.05). I NREM delta spectral power during dark phase in EKyn, sexes combined (treatment × frequency interaction F(7, 49) = 3.861, P < 0.01). J NREM delta spectral power during light phase in EKyn, sexes combined (treatment effect F(1, 7)= 13.29, P < 0.01, treatment × frequency interaction F(7, 49) = 3.050, P < 0.01). Data are mean ± SEM. Percent change from vehicle treatment calculations are shown by arrows. Two-way RM ANOVA: ^P < 0.05, ^^P < 0.01 with Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. N = 8 per group.
Treatment of EKyn offspring at the start of the dark phase with PF-04859989 produced similar impacts as in ECon. PF-04859989 treatment at ZT 12 in EKyn rats elevated NREM duration by 17% and decreased wake duration by 29% during the first half of the consecutive light phase without altering total REM duration (Fig. 4F–H). Sleep architecture, namely the number of NREM and wake bouts, was significantly impacted with PF-04859989 treatment (Supplementary Fig. 3). In contrast to dark phase PF-04859989 treatment in ECon offspring, NREM delta power was significantly impacted when EKyn offspring were treated with PF-04859989 at ZT 12, noted as a statistically significant treatment x frequency interaction throughout both dark and light phases (Fig. 4I, J).
Administration of KAT II inhibitor impacts sleep-wake transitions
Changes in sleep architecture were further assessed by evaluating transitions between vigilance states. PF-04859989 treatment increased the number of REM to NREM transitions when the drug was administered at ZT 0. With treatment at ZT 12, the number of transitions from wake to NREM sleep and conversely from NREM sleep to wake were significantly increased with PF-04859989 (Table 1). The elevated number of transitions from NREM to wake supports our findings that the number of wake bouts is increased by PF-04859989 treatment without a change in the number of NREM bouts (Supplementary Fig. 3). Sleep onset latency, evaluating sleep propensity [47], was unchanged within the immediate light or dark phase after PF-04859989 treatment, however REM onset was earlier during the subsequent dark phase with ZT 0 treatment (Table 1).
Sex-dependent novelty-induced exploration
EKyn offspring pose sex-dependent patterns in behavior including reduced home cage activity [38]. To further characterize these phenotypes, we evaluated basal locomotion and exploration in the open field during both light and dark phases in both sexes of offspring. Exposure to the open field arena induced novelty-induced exploration in a phase-dependent manner. Regardless of prenatal treatment, females had increased ambulation (Fig. 5A), and higher average velocity (Fig. 5B) in comparison to males across 24 h. Of note, exploration of the center of the arena was significantly greater during dark phase compared to light phase testing in males (Fig. 5C). Similarly, the percent of time spent in the corners of the arena was significantly lower during dark phase compared to light phase testing, in both sexes and prenatal conditions (Fig. 5D). We did not determine any EKyn-dependent deficits in open field exploration across measures, however female EKyn spent more time exploring the center of the testing arena compared to EKyn males during the light phase.
Male and female offspring were tested in the open field test during the light or dark phase: A distance traveled, B velocity, C percent of time spent in center, D percent of time spent in corners. Adult ECon and EKyn offspring were treated with vehicle or PF-04859989 (30 mg/kg) at Zeitgeber time (ZT) 0. E 1-h bins of relative cage activity, sexes combined (three-way ANOVA: treatment × ZT interaction F(23, 487) = 1.825, P < 0.05). F 6-h bins of relative cage activity in ECon, sexes combined (treatment × ZT interaction F(3, 39) = 5.621, P < 0.01). G 6-h bins of relative cage activity in EKyn, sexes combined (treatment × ZT interaction F(3, 43) = 3.175, P < 0.05). H 1-h bins of core body temperature, sexes combined (three-way ANOVA: treatment effect F(1, 29) = 9.403, P < 0.01, treatment × ZT interaction F(23, 480) = 2.978, P < 0.0001). I 6-h bins of core body temperature in ECon, sexes combined (treatment effect F(1, 14) = 7.810, P < 0.05). J 6-h bins of core body temperature in EKyn, sexes combined (treatment effect F(1, 15) = 7.507, P < 0.05, treatment × ZT interaction F(3, 43) = 6.280, P < 0.01). Data are mean ± SEM. Percent change from vehicle treatment calculations are shown by arrows. Three-way ANOVA: #P < 0.05, ##P < 0.01, ####P < 0.0001. Two-way RM ANOVA: ^P < 0.05, ^^P < 0.01 with Fisher’s LSD post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. N = 11–16 per group.
Reduced cage activity and body temperature with PF-04859989 treatment
Active wakefulness, characterized by limb movements and locomotor activity, differs from quiescent wake behavior [48]. PF-04859989 administered at the beginning of the light phase significantly impacted cage activity, noted as a treatment x ZT interaction (Fig. 5E). Relative cage activity was decreased after PF-04859989 in both prenatal groups during the second half of the dark phase (Fig. 5F, G). Rats typically exhibit elevated core temperature and increased motor activity during the dark phase [49]. We found that PF-04859989 also impacted core body temperature, noted as an interaction between treatment and ZT (Fig. 5H). In ECon rats, body temperature decreased only in the latter half of the light phase (Fig. 5I), whereas in EKyn offspring the decrease in core body temperature was sustained between ZT 6 and ZT 18 (Fig. 5J). Treatment with PF-04859989 at the beginning of the dark phase produced no significant differences in relative cage activity or core body temperature (Supplementary Fig. 4).
Enhanced improvements in sleep architecture with PF-04859989 treatment at the start of the light phase
To compare the effectiveness of PF-04859989 administration between the start of the light or dark phase, we evaluated the absolute changes in duration of the three vigilance states. Notably, during the first 12 h post-drug administration, we noted stark differences in the enhancement of REM duration (Supplementary Fig. 6A). Changes in NREM duration and wake duration, induced by PF-04859989 treatment, were also impacted by the time of treatment, but major distinctions were found 12 h after drug administration (Supplementary Fig. 6B, C). Absolute changes in NREM delta spectral power were also evaluated, comparing the first 12 h after PF-04859989 treatment and the subsequent 12 h. We observed consistent improvement in NREM power spectrum within the first 12 h regardless of PF-04859989 administration at ZT 0 or ZT 12 (Supplementary Fig. 6D). Spectral power, in the lowest delta frequencies between 0.5–2 Hz, was notably differentially impacted between 12 and 24 h after PF-04859989 administration (Supplementary Figure 6E).
Discussion
Our present study is the first to signify that acute KAT II inhibition improves sleep physiology and architecture, thereby suggesting that reducing brain KYNA alleviates sleep disturbances. KAT II inhibitor PF-04859989 effectively enhanced sleep parameters in both ECon and EKyn offspring of both sexes. Our findings contribute to the recent growing body of literature showing that the pivotal enzymes within the KP serve as effective physiological treatment targets [10, 50].
Tryptophan metabolism has long been implicated in modulating sleep, namely through its formation of the circadian hormone melatonin [51, 52]. Yet, a role for KP metabolites, specifically KYNA, in regulating sleep-wake behavior has only recently been discovered. Acutely elevating de novo production of brain KYNA showed prompt reduction in REM sleep, including alterations in REM architecture [41]. Similarly, REM sleep dynamics are disrupted in young adult EKyn offspring, wherein elevated brain KYNA levels are postulated to disrupt sleep and arousal patterns compared to counterpart controls. EKyn males have reduced duration of REM sleep concomitant with failing to initiate REM episodes, while the number of both NREM and wake bouts was lower in EKyn females compared to controls [38]. We presently leverage the strengths of our EKyn preclinical model to test the novel hypothesis that reducing brain KYNA can impact sleep duration and quality, and aim to place further attention on the KP as a targetable metabolic pathway to improve sleep homeostasis.
KYNA is an endogenous antagonist of the glutamatergic N-methyl-D-aspartate (NMDA) receptors and alpha-7 nicotinic acetylcholine receptors (α7nAChR)[16, 53]. Cholinergic transmission is mediated by activation of the presynaptic α7nAChRs on glutamatergic neurons and the postsynaptic α7nAChRs on parvalbumin GABA (gamma-aminobutyric acid)-ergic interneurons [54, 55]. Decrease in KYNA levels is postulated to physiologically enhance cholinergic signaling across the brain and stimulate release of glutamate [15]. As the generation of REM sleep and sleep stability is promoted by excitatory glutamatergic neurotransmission [48, 56], we can presently speculate that global reduction in KYNA contributes to the stability of REM sleep. In the EKyn rats, extracellular glutamate levels are reduced during the light phase [35], and KAT II inhibition effectively restores glutamate to control levels [37]. Further exploring circuit level modulation from the brainstem pontine areas to the cerebral cortex and the hippocampus to regulate REM sleep stability is warranted in future studies [57,58,59].
Demand for novel, efficacious sleep therapeutics has increased substantially in the recent years. Treatments that improve sleep onset latency, maintenance, efficiency, slow wave power, and overall sleep quality could ameliorate commonly reported sleep problems in SCZ and BPD patients [60,61,62,63,64,65,66,67,68,69]. Unfortunately, many commonly prescribed antipsychotic drugs, along with adjunct antidepressant therapeutic approaches, suppress sleep further [70, 71]. Our present findings are translationally noteworthy as KAT II inhibition effectively improves sleep outcomes in both EKyn, our neurodevelopmental insult paradigm, and the counterpart controls, ECon. Single administration of KAT II inhibitor was sufficient to elicit recovered sleep architecture in EKyn rats to the levels that are observed in ECon rats for the subsequent 24-h period. Evaluation of the time of day of PF-04859989 treatment concluded that treatment at the beginning of the light phase triggers immediate improvements in sleep behavior, while treatment at the beginning of the dark phase deferred outcomes until the subsequent light phase. Notably, the ability of PF-04859989 to maintain entrainment of the light-dark cycle in adult rodents further supports the translational potential of pharmacological approaches targeting KAT II.
In addition to promoting sleep duration, the KAT II inhibitor enhanced NREM delta power, potentially advancing overall sleep quality. Delta spectral power indicates homeostatic sleep drive, but it is regulated independently of sleep duration [72]. Delta waves of NREM sleep occur when neurons originating from extensive areas of the cortex repeatedly transit between a hyperpolarized and depolarized state [73, 74]. These slow cortical oscillations couple and synchronize with sharp-wave ripples generated in the hippocampus to facilitate memory consolidation and various cognitive functions [75,76,77]. PF-04859989 treatment enhanced NREM spectral power dynamically in a sex- and phase-dependent manner presently. This finding is informative for the development of targeted therapeutic strategies to enhance sleep quality, and in tandem have the potential to enhance cognitive function [25].
Cognitive deficits and memory impairments are lingering symptoms that persist in individuals with severe psychiatric illnesses albeit antipsychotic therapy [78,79,80,81]. Clinical studies also point to sex differences in cognitive performance in individuals with SCZ. Notably across several studies, cognitive deficits in males are more adverse and their response to various antipsychotic medications is less effective [82,83,84,85,86,87]. Our EKyn model is highly translationally relevant, as we have determined sex differences in cognitive outcomes that align with the aforementioned clinical findings. Male EKyn offspring are more adversely impacted in learning and cognitive flexibility in behavioral tasks compared to female offspring [32]. KAT II inhibitor PF-04859989 promotes sleep quality, irrespective of sex, and thus we speculate that PF-04859989 treatment of EKyn offspring may also effectively improve cognitive performance, as in behavioral experiments with KAT II inhibitor BFF-816 in EKyn offspring [37].
Sleep quality may also contribute to avolition, considered a core negative symptom in individuals with SCZ and BPD [88,89,90,91]. To evaluate if sleep disturbances contribute to a lack of motivation, slow movement, and fatigue, exploratory and locomotor behaviors were evaluated, yet we determined no differences between ECon and EKyn offspring. We did, however, note higher mobility in female rats, as reported by others [92]. Female behavioral and physiological processes, including cognition and sleep, are greatly impacted by the hormonal status and the estrous cycle [93, 94] and the presentation of symptoms in females with SCZ is also often at a later age than males [95]. Presently, our limitation is a lack of comprehensive knowledge of hormonal status in our female subjects. Vaginal cytology was not collected on the drug treatment days to minimize handling stress, yet in an effort to match estrous cycle stages within subjects, vehicle and PF-04859989 treatments were separated by 4 days in females [96]. To comprehensively define a role for KYNA in the timeline of sleep and affective symptoms, future preclinical and clinical studies will be imperative [91, 97, 98].
In closing, we presently place critical attention on a novel targeted therapeutic strategy for overcoming sleep disorders. Acute KAT II inhibition to reduce KYNA is shown to enhance REM and NREM sleep, and restore sleep stability in EKyn, our neurodevelopmental insult model. Increased NREM delta power with KAT II inhibition may also influence the quality and architecture of sleep, and future studies will need to critically evaluate how the homeostatic process of sleep may be altered in subsequent days as well. Improvement in sleep stability places much needed attention on a targeted approach which may lead to the development of potential therapeutic interventions in individuals combatting sleep disruptions alongside neuropsychiatric and neurodevelopmental disorders.
References
Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17:299–313.
Laskemoen JF, Simonsen C, Buchmann C, Barrett EA, Bjella T, Lagerberg TV, et al. Sleep disturbances in schizophrenia spectrum and bipolar disorders - a transdiagnostic perspective. Compr Psychiatry. 2019;91:6–12.
Alachkar A, Lee J, Asthana K, Vakil Monfared R, Chen J, Alhassen S, et al. The hidden link between circadian entropy and mental health disorders. Transl Psychiatry. 2022;12:281.
Krystal AD. Psychiatric disorders and sleep. Neurol Clin. 2012;30:1389–413.
Sutton EL. Psychiatric disorders and sleep issues. Med Clin N Am. 2014;98:1123–43.
Palagini L, Manni R, Aguglia E, Amore M, Brugnoli R, Bioulac S, et al. International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders. Front Psychiatry. 2021;12:688890.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307–49.
Liu MT. Current and emerging therapies for insomnia. Am J Manag Care. 2020;26:S85–S90.
Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549–66.
Muneer A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clin Psychopharmacol Neurosci. 2020;18:507–26.
Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237–47.
Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938.
Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794.
Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol. 2013;85:1027–32.
Bortz DM, Wu HQ, Schwarcz R, Bruno JP. Oral administration of a specific kynurenic acid synthesis (KAT II) inhibitor attenuates evoked glutamate release in rat prefrontal cortex. Neuropharmacology. 2017;121:69–78.
Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73.
Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8.
Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-4:25–37.
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30.
Sellgren CM, Kegel ME, Bergen SE, Ekman CJ, Olsson S, Larsson M, et al. A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol Psychiatry. 2016;21:1342–50.
Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2020;25:2860–72.
Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997;50:457–65.
Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109:316–25.
Jayawickrama GS, Sadig RR, Sun G, Nematollahi A, Nadvi NA, Hanrahan JR, et al. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Curr Med Chem. 2015;22:2902–18.
Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–602.
Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112:297–306.
Notarangelo FM, Pocivavsek A. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology. 2017;112:275–85.
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology. 2014;231:2799–809.
Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83.
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-7:1–16.
Buck SA, Baratta AM, Pocivavsek A. Exposure to elevated embryonic kynurenine in rats: sex-dependent learning and memory impairments in adult offspring. Neurobiol Learn Mem. 2020;174:107282.
Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41.
Pershing ML, Phenis D, Valentini V, Pocivavsek A, Lindquist DH, Schwarcz R, et al. Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology. 2016;233:3725–35.
Wright CJ, Rentschler KM, Wagner NTJ, Lewis AM, Beggiato S, Pocivavsek A. Time of day-dependent alterations in hippocampal kynurenic acid, glutamate, and GABA in adult rats exposed to elevated kynurenic acid during neurodevelopment. Front Psychiatry. 2021;12:734984.
Hahn B, Reneski CH, Pocivavsek A, Schwarcz R. Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood. Psychopharmacology. 2018;235:651–61.
Pocivavsek A, Elmer GI, Schwarcz R. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus. 2019;29:73–7.
Rentschler KM, Baratta AM, Ditty AL, Wagner NTJ, Wright CJ, Milosavljevic S, et al. Prenatal kynurenine elevation elicits sex-dependent changes in sleep and arousal during adulthood: implications for psychotic disorders. Schizophr Bull. 2021;47:1320–30.
Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365:547–9.
Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12.
Pocivavsek A, Baratta AM, Mong JA, Viechweg SS. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep. 2017;40:zsx141.
Smith A, Anand H, Milosavljevic S, Rentschler KM, Pocivavsek A, Valafar H. Application of machine learning to sleep stage classification. In: 2021 International Conference on Computational Science and Computational Intelligence. Las Vegas, NV, USA: Int Conf Comput Sci Comput Intell; 2021. p. 349–54.
Klausing AD, Fukuwatari T, Bucci DJ, Schwarcz R. Stress-induced impairment in fear discrimination is causally related to increased kynurenic acid formation in the prefrontal cortex. Psychopharmacology. 2020;237:1931–41.
Long S, Ding R, Wang J, Yu Y, Lu J, Yao D. Sleep quality and electroencephalogram delta power. Front Neurosci. 2021;15:803507.
Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352:812–6.
Boyce R, Williams S, Adamantidis A. REM sleep and memory. Curr Opin Neurobiol. 2017;44:167–77.
McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–73.
Datta S, Hobson JA. The rat as an experimental model for sleep neurophysiology. Behav Neurosci. 2000;114:1239–44.
Hankenson FC, Marx JO, Gordon CJ, David JM. Effects of rodent thermoregulation on animal models in the research environment. Comp Med. 2018;68:425–38.
Bohar Z, Toldi J, Fulop F, Vecsei L. Changing the face of kynurenines and neurotoxicity: therapeutic considerations. Int J Mol Sci. 2015;16:9772–93.
Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G. Melancholic-Like Behaviors and Circadian Neurobiological Abnormalities in Melatonin MT1 Receptor Knockout Mice. Int J Neuropsychopharmacol. 2015;18:pyu075.
Gobbi G, Comai S. Differential function of melatonin MT1 and MT2 receptors in REM and NREM sleep. Front Endocrinol. 2019;10:87.
Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–28.
Krenz I, Kalkan D, Wevers A, de Vos RA, Steur EN, Lindstrom J, et al. Parvalbumin-containing interneurons of the human cerebral cortex express nicotinic acetylcholine receptor proteins. J Chem Neuroanat. 2001;21:239–46.
Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–8.
Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–88.
Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–9.
Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007;584:735–41.
Lopez-Rodriguez F, Medina-Ceja L, Wilson CL, Jhung D, Morales-Villagran A. Changes in extracellular glutamate levels in rat orbitofrontal cortex during sleep and wakefulness. Arch Med Res. 2007;38:52–5.
Chan MS, Chung KF, Yung KP, Yeung WF. Sleep in schizophrenia: a systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2017;32:69–84.
Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–67.
Faulkner S, Sidey-Gibbons C. Use of the Pittsburgh sleep quality index in people with schizophrenia spectrum disorders: a mixed methods study. Front Psychiatry. 2019;10:284.
Ferrarelli F. Sleep in patients with schizophrenia. Curr Sleep Med Rep. 2015;1:150–6.
Gold AK, Sylvia LG. The role of sleep in bipolar disorder. Nat Sci Sleep. 2016;8:207–14.
Gruber J, Harvey AG, Wang PW, Brooks JO 3rd, Thase ME, Sachs GS, et al. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Affect Disord. 2009;114:41–9.
Harvey AG, Talbot LS, Gershon A. Sleep disturbance in bipolar disorder across the lifespan. Clin Psychol. 2009;16:256–7.
Roloff T, Haussleiter I, Meister K, Juckel G. Sleep disturbances in the context of neurohormonal dysregulation in patients with bipolar disorder. Int J Bipolar Disord. 2022;10:6.
Steardo L Jr., de Filippis R, Carbone EA, Segura-Garcia C, Verkhratsky A, De Fazio P. Sleep disturbance in bipolar disorder: neuroglia and circadian rhythms. Front Psychiatry. 2019;10:501.
Zanini MA, Castro J, Cunha GR, Asevedo E, Pan PM, Bittencourt L, et al. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;169:262–7.
McCarthy A, Wafford K, Shanks E, Ligocki M, Edgar DM, Dijk DJ. REM sleep homeostasis in the absence of REM sleep: Effects of antidepressants. Neuropharmacology. 2016;108:415–25.
Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51–7.
Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–8.
Siclari F, Bernardi G, Riedner BA, LaRocque JJ, Benca RM, Tononi G. Two distinct synchronization processes in the transition to sleep: a high-density electroencephalographic study. Sleep. 2014;37:1621–37.
Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106.
Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30.
Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70.
Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100:2065–9.
Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2:531–6.
Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–6.
Sharma T, Antonova L. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin N Am. 2003;26:25–40.
Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009;11:113–25.
Carrus D, Christodoulou T, Hadjulis M, Haldane M, Galea A, Koukopoulos A, et al. Gender differences in immediate memory in bipolar disorder. Psychol Med. 2010;40:1349–55.
Han M, Huang XF, Chen DC, Xiu MH, Hui L, Liu H, et al. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:358–63.
Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631.
Usall J, Suarez D, Haro JM, Group SS. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153:225–31.
Xu X, Xiang H, Qiu Y, Teng Z, Li S, Huang J, et al. Sex differences in cognitive function of first-diagnosed and drug-naive patients with bipolar disorder. J Affect Disord. 2021;295:431–7.
Zhang B, Han M, Tan S, De Yang F, Tan Y, Jiang S, et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci Rep. 2017;7:11821.
Strassnig MT, Miller ML, Moore R, Depp CA, Pinkham AE, Harvey PD. Evidence for avolition in bipolar disorder? A 30-day ecological momentary assessment comparison of daily activities in bipolar disorder and schizophrenia. Psychiatry Res. 2021;300:113924.
Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–90.
Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:88–92.
Mukherjee D, Krishnamurthy VB, Millett CE, Reider A, Can A, Groer M, et al. Total sleep time and kynurenine metabolism associated with mood symptom severity in bipolar disorder. Bipolar Disord. 2018;20:27–34.
Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69.
Djiogue S, Djiyou Djeuda AB, Seke Etet PF, Ketcha Wanda GJM, Djikem Tadah RN, Njamen D. Memory and exploratory behavior impairment in ovariectomized Wistar rats. Behav Brain Funct. 2018;14:14.
Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16.
Goldstein JM, Cherkerzian S, Tsuang MT, Petryshen TL. Sex differences in the genetic risk for schizophrenia: history of the evidence for sex-specific and sex-dependent effects. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:698–710.
Lovick TA, Zangrossi H Jr. Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front Psychiatry. 2021;12:711065.
Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res. 2017;99:1–7.
Mori Y, Mouri A, Kunisawa K, Hirakawa M, Kubota H, Kosuge A, et al. Kynurenine 3-monooxygenase deficiency induces depression-like behavior via enhanced antagonism of alpha7 nicotinic acetylcholine receptors by kynurenic acid. Behav Brain Res. 2021;405:113191.
Acknowledgements
The authors would like to thank Audrey Ditty, Katherine Rentschler, and Nathan Wagner for their technical contributions to this work. This research was supported by National Institutes of Health Grant Nos. NIH R01 NS102209 (AP), P50 MH103222 (AP), and P20 RR-016461 (HV).
Author information
Authors and Affiliations
Contributions
SM: Conducted research, formal analysis, writing – original draft, writing – review & editing, visualization, project administration. AKS: Conducted research, methodology, formal analysis, writing – review & editing, visualization. CJW: Conducted research, methodology, formal analysis, writing – review & editing. HV: Methodology, formal analysis, writing – review & editing, supervision, project administration, funding acquisition. AP: Conceptualization, methodology, formal analysis, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Milosavljevic, S., Smith, A.K., Wright, C.J. et al. Kynurenine aminotransferase II inhibition promotes sleep and rescues impairments induced by neurodevelopmental insult. Transl Psychiatry 13, 106 (2023). https://doi.org/10.1038/s41398-023-02399-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02399-1
This article is cited by
-
Kynurenic acid: translational perspectives of a therapeutically targetable gliotransmitter
Neuropsychopharmacology (2024)