Abstract
Aerobic exercise is effective in alleviating mood symptoms while the mechanism is poorly understood. There are limited clinical trials that investigated the effect of exercise on the anterior cingulate cortex (ACC), a key brain region involved in mood regulations, in adolescents with subthreshold mood syndromes. This randomized controlled trial (RCT) of aerobic exercise was undertaken in a middle school in Guangzhou, China. Participants were adolescents aged 12–14 with subthreshold mood syndromes including depressive and manic symptoms and were randomly assigned to an aerobic exercise intervention or a psychoeducation control group. Participants in the exercise group received moderate-intensity exercise intervention, consisting of 30 mins running, 4 days per week for 3 months. The primary outcome in this study was structural changes in the ACC from baseline to post intervention. The trial was registered with ClinicalTrial.gov (NCT03300778). Of 56 participants who met the criteria for subthreshold mood syndromes, 39 (41.03% males) had complete MRI data, with 20 and 19 subjects in the exercise and control group, respectively. At baseline, demographic information (e.g., age and sex), clinical symptoms, and the gray matter volume and cortical thickness of ACC were matched between the two groups. After 12 weeks of treatment, participants in the exercise group displayed increased gray matter volume of the left rostral ACC (F1,30 = 5.73, p = 0.02) and increased cortical thickness of the right rostral ACC (F1,30 = 7.83, p = 0.01) when compared with the control group. No significant differences were found for caudal ACC cortical thickness and gray matter volume. Our data demonstrate that 12-week, moderate-intensity aerobic exercise can induce structural changes in the rostral ACC in adolescents with subthreshold mood syndromes.
Similar content being viewed by others
Introduction
At least 50% of mental health symptoms and disorders develop in youth and adolescence1. The burden of poor mental health and wellbeing remains substantial during this key time of transition2. Early intervention during adolescence is key in order to effectively treat the mental health symptoms and prevent any longer term impact during later life3.
Mood disorders are a leading cause of mental health burden in adolescence3. A number of different treatment approaches have been proposed such as antidepressant medication4, psychological therapy5, and psychoeducation6. However, antidepressant medication may have side effects7 and possibly be related increased risk of suicide attempts8. Psychological therapy does not work for all adolescents9. In addition, there is increasing recognition that mood disorders in adolescence often co-occur with physical health comorbidity such as obesity and diabetes10,11,12 which medication and psychological therapy do not address. Moreover, mood disorders are often associated with impaired cognitive capabilities, which can impede adolescents functioning during this critical time and treatment options are limited10,12. Furthermore, adolescents who presented subthreshold depression already displayed abnormalities in the white matter tracts connecting the corpus callosum to the anterior cingulate cortex (ACC), which was associated with higher risk for developing depressive disorder13.
Recently, there has been a dramatic rise in interest for the potential for physical exercise to prevent and manage mood disorders in adolescence14,15,16,17. Physical exercise has been established to have a key role in promoting positive physical health in adolescence such as reduced risk of physical comorbidities18. In addition, robust evidence indicates that exercise can improve neurocognition, academic performance, and induce structural changes in the adolescence brain19,20. Recently, some systematic review have suggested that exercise can improve mental health symptoms in adolescence21,22 with subthreshold and established mood disorders.
Although some progress has been made demonstrating the efficacy of exercise, limited clinical trials have explored the potential underlying mechanisms, particularly in adolescence23. A few clinical trials in older adults with or without cognitive impairment showed that ACC is a potential target to be changed by exercise24. The ACC serves as a hub for global neural network dynamics underlying an array of functions, e.g., mood regulations, cognitive, and social function25,26. Aberrant development of the ACC in adolescents has been implicated in mood disorders including depression and bipolar disorder27,28. However, few randomized controlled trial (RCT) studies by far have investigated the exercise effects on adolescents with subthreshold mood symptoms, a high-risk population in developing mood disorders1.
Given the above considerations, the aim of this study was to investigate the effects of 12-week moderate-intensity aerobic exercise on the ACC structure. We hypothesized that the aerobic exercise could increase gray matter volume and cortical thickness of the ACC.
Methods
Study design and participants
This exercise study is a prospective, two-arm, parallel-group, randomized, controlled trial (NCT03300778). Participants were recruited from a middle school in Guangzhou, China. The study was approved by the IRB of The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital). All the participants and their guardians provided written informed consent.
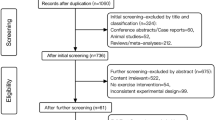
In all, 233 subjects in the first year of middle school (age 12–14 yeas) were invited to participate in the trial. Nine subjects refused and 224 participants completed interviews with a psychiatrist and a psychologist to determine eligibility. We used a self-reported, 74-item symptom checklist and a Chinese version of the Bipolar Prodrome Scale-Retrospective: Patient Version (BPSS-R-Pt) to assess current and pass symptoms respectively, based on which research psychiatrists using the DSM-5 criteria to exclude psychiatric disorders. In all, 56 participants with subthreshold mood syndromes were confirmed using the following definition. They either presented (i) subthreshold depression defined as two or more depressive symptoms lasting for at least 1 week but falling short of the criteria for a major depressive episode, or (ii) subthreshold hypomania defined as two or more (up to four if mood was only irritable) manic symptoms lasting at least 4 days or as meeting the hypomanic symptom criteria lasting 2–3 days. The participants of this study were not on any psychiatric medications. Any DSM-4-defined psychiatric disorders were excluded. Other exclusions were physical diseases that were not suitable for running exercise (e.g., cardiovascular and neurological diseases), severe suicidal ideation, mental retardation, and currently having been involved in any formal exercise training programs. All of the participants with subthreshold mood syndromes were invited to do MRI scanning and 39 of 56 (69.6%) completed.
Randomization and procedures
Randomization code was generated by a specially designed procedure. Randomization was performed in a 1:1 ratio, and the exercise intervention assignment was not masked. But data collectors were told not to ask participant’s study assignment and primary investigators were blinded to allocation. We applied general psychoeducation as a parallel, controlled arm in order to balance potential placebo effect of being treated as it might be seen in the exercise running group. Outcome assessors were instructed not to ask participants’ treatment allocation and were blind to the treatment allocation.
The aerobic exercise group received moderate-intensity running, which was defined as 50–70% of maximum heart rate (calculated as 220-age, beats/minute), 30 min per day, 4 days per week for a total of 3 months. The exercise took place in the school playgroup in the afternoon after class. Each participant’ heart rate was measured with a heart rate monitor right before and after the 30-min running exercise. Because all the participants were in a similar age, a volunteer lead participant in each team that is consisted of ~30 participants helped to meet the exercise intensity (50–70% of maximum heart rate) by adjusting his/her speed. The whole exercise process was video-recorded each time. Approximately 88% participants in the exercise intervention arm finished >80% of the exercise sections.
The control group received a namely psychoeducation treatment, which consisted of three sections of general psychological education, one section of group game, poetry reading, and singing. Approximately 90% participants attended four or more out of six sections.
Primary outcome measures
The co-primary outcomes were gray matter (GM) volumes and cortical thickness of the ACC (i.e., rostral and caudal).
Structural imaging data acquisition and processing
Structural imaging data were acquired using a Philips Achieva X-series 3.0 T scanner equipped with an eight-channel SENSE head coil. The scan parameters were described as below: the parameters were: repetition time (TR) = 8.2 ms; echo time (TE) = 3.7 ms; FOV = 256× 256 mm2; voxel sizes = 1 × 1 × 1 mm3; matrix size = 256 × 256; slices = 188; slices thickness =1 mm; slices gaps = 0 mm.
MRI data preprocessing and analysis
A quality check for motion and dropout artifacts was conducted before the structural MRI data were preprocessed. Structural MRI data including volumetric segmentation were preprocessed using FreeSurfer software (FreeSurfer 4.0.5, http://surfer.nmr.mgh.harvard.edu). The following preprocessing steps were conducted to estimate the GM in cortical and subcortical areas: motion correction, non-parametric non-uniform intensity correction, transformation of the original volume to the MNI305 atlas using the MINC program MRITOTAL, intensity normalization, skull stripping, automatic subcortical segmentation, GCA atlas registration, removal of neck region, EM registration with the skull, CA labeling (labeling of subcortical structures using the GCA model), white matter segmentation, and cutting of the mid- brain from the cerebrum, and the hemispheres from each other. No manual corrections of the automaged outputs from FreeSurfer were performed. Upon completion of all preprocessing, analyses of the primary ROIs were conducted using SPSS 24 (Chicago, IL, USA).
Statistical analysis
Participants’ demographic and clinical variables were analyzed using independent-sample t test or Chi-square test. Research of interest (ROI) analyses were performed for the ACC GM volume and cortical thickness, including rostral ACC and caudal ACC. The ROI GM volumes and cortical thickness across Time (baseline and follow-up) and Group (exercise intervention group and control group) as well as Group × Time interaction effect were investigated. By testing the Group × Time interaction effect, we tested whether there were neural changes across time, which interacted with the group effect. Specifically, we investigated whether there were significant structural neural changes after the exercise intervention but not the control group. Therefore, the neural correlates were the dependent variables and Group was the between-group independent variable while Time was the within-group independent variable in the present study.
Analyses of the ROI neural correlates were performed using SPSS (Chicago, IL, USA) via employing repeated measure t test analysis. Repeated measure t test analysis was conducted for the structural neural correlates with Group, Time, and the interaction term of the two as the independent variables. Significance was set based on a two-tailed alpha level of 0.05 for all tests. In all repeated measure t test analyses with neural correlates as the dependent variables, the whole-brain volumes were controlled for.
Results
In all, 223 students were assessed for eligibility, and 56 met the criteria for subthreshold mood syndromes and were randomly assigned to aerobic exercise or psychoeducation controlled. In all, 39 of the 56 participants completed the MRI scan. In total, 20 and 19 subjects were in the exercise and control group, respectively. Baseline demographic information, clinical characteristics, and neural correlates were balanced between the two groups (Table 1). There was no difference in the effect of exercise versus psychoeducation (ps > 0.05).
Exercise effects on the brain structure
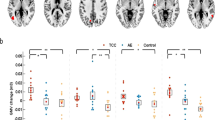
The repeated- measures t test results showed that the Group × Time interaction effect was significant (F1,30 = 5.73, p = 0.02), whereas the Time (F1,30 = 3.75, p = 0.06) and Group effect (F1,30 = 0.17, p = 0.68) were not significant in the left ACC GM volume (Fig. 1). Either Group × Time interaction effects or main effects were not significant in the right rostral ACC GM volume (ps > 0.05). For both the left and right caudal ACC GM volume, either Group × Time interaction effects or main effects were not significant (ps > 0.05).
As to the right rostral ACC cortical thickness, the Group × Time interaction effect was significant (F1,30 = 7.83, p = 0.01) (Fig. 2). Although the Time (F1,30 = 0.12, p = 0.73), Group effect (F1,30 = 0.17, p = 0.68) were not significant. Either Group × Time interaction effects or main effects were not significant in the left rostral ACC cortical thickness (ps > 0.05). For both left and right caudal ACC cortical thickness, either Group × Time interaction effects or main effects were not significant (ps > 0.05).
Discussion
To the best of our knowledge, this is the first RCT to investigate structural brain changes in adolescents with subthreshold mood disorders. In our study, we identify potential changes in key brain areas implicated in mood disorders, which may improve in response to 12-week moderate-intensity aerobic exercise, i.e., GM volume of the left rostral ACC and cortical thickness of the right rostral ACC.
Previous literature has confirmed that exercise can help for subthreshold and established mood disorders, yet the mechanisms are poorly understood23. The ACC investigated in this study have been identified as being impacted and reduced in adolescent with subthreshold depression and hypomania13,29. Relatively mild mood fluctuations in the forms of subthreshold depression and hypomania particularly during adolescence are core features of disturbances in mood regulation30,31. Our sample comprised of adolescents manifesting both subthreshold depression and hypomania. Despite concrete mood symptoms, the IMAGEN study showed that both adolescents with subthreshold depression and bipolar symptoms had decreased structural volumes in the ACC32,33. ACC is postulated as a hub in the brain networks34,35 and implicated in neurocognitive function and reward learning and decision-making36 in addition to conflict monitoring37 and social processing38. Moreover, adolescents with subthreshold mood syndromes are at higher risk of developing mood disorders30,39. Adolescents with depression had decreased negative connectivity between the rostral ACC and the bilateral amygdala, suggesting deficient control of the rostral ACC over amygdala40. Furthermore, the top–down control of ACC over the amygdala has been suggested to be an important feature of pathopsychology in bipolar disorder28.
Patients with bipolar disorderat the very early illness course were also found to be have decreased structural volume of ACC41,42. Its metabolic changes were reported prior to depression43. We previously reported that individuals with subthreshold mood syndromes and a family history of bipolar disorder displayed a disregulated correlation between rostral ACC and the level of IL-6 and attention function44 when compared with those without symptoms.
Clinical trials of exercise (and increased physical activity) that have investigated the exercise effects on the ACC were limited and are mainly on elderly individuals with or without cognitive impairments24,45. For instance, Ruscheweyh et al., assigned a group of 62 elderly healthy individuals into medium-intensity exercise (nordic walking, n = 21), low-intensity exercise (gymnastics, n = 20) or no intervention (control, n = 20). The two exercise groups were asked to exercise for 50 mins, three times per week for a total of 6 months. They found that the changes in the GM volume of the left ACC was correlated with physical activity24. A clinical study of Baduanjin—a type of mind-body exercise—found that Baduanjin increased activity in the ACC at rest in patients with mild cognitive impairment45. Another clinical trial on older individuals (average age = 70) also found that 6 months of progressive resistance physical training could increase GM volume in the posterior ACC46. Given that increasing evidence showing individuals with subthreshold depression and hypomania and patients with bipolar disorder displayed decreased ACC structure compared with the health individuals, and that exercise can help ameliorate depressive symptoms in adolescents47, we speculate that the increased GM volume of the ACC might be beneficial to individuals with subthreshold mood symptoms. Nonetheless, we did not find that the changes of ACC were positively associated with that of clinical symptoms and neurocognition. Given the role of ACC as a key region in mood regulation, future study needs to investigate whether the change of ACC including structural and functional change could impact other behavioral measures related to mood regulation so as to elucidate the mechanism of exercise-induced change of the ACC in individuals with subthreshold mood symptoms.
It is worth noting that we did not find any exercise effect on the clinical symptoms (e.g., PHQ-9) and neurocognitive function (measured by the MATRICS Consensus Cognitive Battery, MCCB) (the results owing to be reported elsewhere). The changes of the ACC structure were not correlated with that of clinical symptoms and of neurocognition (data not shown). It is possible that the changes in the rostral ACC may not be sufficient to cause measurable behavioral or symptom changes. Moreover, as these individuals’ symptoms (including depressive and hypomanic) and cognition were not severely impaired at baseline, the sample size of our trial may not have enough statistical power to detect clinically meaningful difference.
Strengths and limitations
This exercise trial was well conducted in the school playground and video-recorded. The exercise protocol including exercise intensity and duration was strictly followed. Participants had high attendance. Although there were some limitations that should be noted when interpreting our findings. First, the sample size was relatively small (n = 39). As a consequence, it may not have the statistical power to detect exercise effects on the behavioral measures, which could help appreciate clinical implications of the enhanced structural changes identified. Second, we only included adolescents aged between 12 and 14 years and the finding may not be applied to older adolescents. Third, the trial only applied running exercise. It is not clear whether the exercise effect on the ACC structure could be generalized to other types of exercise. Future study needs to compare the effects of different types of exercise on the brain structure. Fourth, the exercise trial was short-term. It is not clear whether the structural changes identified could affect the adolescents’ long-term outcomes. Finally, in this 6-week trial, we did not follow-up those high-risk adolescents for developing mood disorders. It is of great interest to the field whether exercise intervention can delay or prevent the onset of mood disorders. Future study need to answer this scientific question.
In conclusion, our data show that 12-week, moderate-intensity aerobic exercise can positively change the rostral ACC structure in adolescents with subthreshold mood syndromes. Given the ACC has a key role in a array of critical functions and its reduced structure is seen at the very early course of mood disorder, our findings support implementation of aerobic exercise in this high-risk population.
References
Patel, V., Flisher, A. J., Hetrick, S. & McGorry, P. Adolescent Health 3: mental health of young people: a global public-health challenge. Lancet 369, 1302 (2007).
Azzopardi, P. S. et al. Progress in adolescent health and wellbeing: tracking 12 headline indicators for 195 countries and territories, 1990–2016. Lancet 393, 1101–1118 (2019).
Davey, C. G. & McGorry, P. D. Early intervention for depression in young people: a blind spot in mental health care. Lancet Psychiatry 6, 267–272 (2019).
Zhou, X. et al. Comparative efficacy and acceptability of antidepressants, psychological interventions, and their combination for depressive disorder in children and adolescents: protocol for a network meta-analysis. BMJ Open. 7, e16608 (2017).
Goodyer, I. M. et al. Cognitive behavioural therapy and short-term psychoanalytical psychotherapy versus a brief psychosocial intervention in adolescents with unipolar major depressive disorder (IMPACT): a multicentre, pragmatic, observer-blind, randomised controlled superiority trial. Lancet Psychiatry 4, 109–119 (2017).
Bevan Jones, R. et al. Psychoeducational interventions in adolescent depression: a systematic review. Patient Educ. Couns 101, 804–816 (2018).
Dragioti, E. et al. Association of antidepressant use with adverse health outcomes. JAMA Psychiatry 76, 1241 (2019).
Sharma T., Guski L. S., Freund N., Gøtzsche P. C. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ 352, i65 (2016).
Weersing, V. R., Jeffreys, M., Do, M. T., Schwartz, K. T. & Bolano, C. Evidence base update of psychosocial treatments for child and adolescent depression. J. Clin. Child Adolesc. Psychol. 46, 11–43 (2017).
Galling, B. et al. Type 2 diabetes mellitus in youth exposed to antipsychotics. JAMA Psychiatry 73, 247 (2016).
Koehler, F. et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 392, 1047–1057 (2018).
Bernaras, E., Jaureguizar, J. & Garaigordobil, M. Child and adolescent depression: a review of theories, evaluation instruments, prevention programs, and treatments. Front. Psychol. 10, 543 (2019).
Phillips, M. L. & Swartz, H. A. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 171, 829–843 (2014).
Vulser, H., PaillèreMartinot, M. L. & Artiges, E. Early variations in white matter microstructure and depression outcome in adolescents with subthreshold depression. Am. J. Psychiatry 12, 1255–1264 (2018).
Schuch, F. B. et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am. J. Psychiatry 175, 631–648 (2018).
Kandola, A., Ashdown-Franks, G., Stubbs, B., Osborn, D. P. J. & Hayes, J. F. The association between cardiorespiratory fitness and the incidence of common mental health disorders: a systematic review and meta-analysis. J. Affect. Disord. 257, 748–757 (2019).
Schuch, F. B. & Stubbs, B. The role of exercise in preventing and treating depression. Curr. Sport. Med. Rep. 18, 299–304 (2019).
Ashdown-Franks, G. et al. Exercise as medicine for mental and substance use disorders: a meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 50, 151–170 (2020).
D. Angelo, H., Fowler, S. L., Nebeling, L. C. & Oh, A. Y. Adolescent physical activity: moderation of individual factors by neighborhood environment. Am. J. Prev. Med. 52, 888–894 (2017).
Singh, A., Uijtdewilligen, L., Twisk, J. W., van Mechelen, W. & Chinapaw, M. J. Physical activity and performance at school. Arch. Pediatr. Adolesc. Med. 1, 49–55 (2012).
Lubans, D. et al. Physical activity for cognitive and mental health in youth: a systematic review of mechanisms. Pediatrics 138, e20161642 (2016).
Carter, T. P., Morres, I. D. M., Meade, O. P. & Callaghan, P. P. The effect of exercise on depressive symptoms in adolescents: a systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 55, 580–590 (2016).
Bailey, A. P., Hetrick, S. E., Rosenbaum, S., Purcell, R. & Parker, A. G. Treating depression with physical activity in adolescents and young adults: a systematic review and meta-analysis of randomised controlled trials. Psychol. Med. 48, 1068–1083 (2018).
Kandola, A., Ashdown-Franks, G., Hendrikse, J., Sabiston, C. M. & Stubbs, B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav Rev. 107, 525–539 (2019).
Ruscheweyh, R. et al. Physical activity and memory functions: an interventional study. Neurobiol. Aging. 32, 1304–1319 (2011).
Shenhav, A., Barrett, L. F. & Bar, M. Affective value and associative processing share a cortical substrate. Cogn., Affect., Behav. Neurosci. 13, 46–59 (2013).
Lichenstein, S. D., Verstynen, T. & Forbes, E. E. Adolescent brain development and depression: a case for the importance of connectivity of the anterior cingulate cortex. Neurosci. Biobehav. Rev. 70, 271–287 (2016).
Cullen, K. R. et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry 49, 173–183 (2010).
LeWinn, K. Z. S. et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J. Am. Acad. Child Adolesc. Psychiatry 53, 899–909 (2014).
Axelson, D. et al. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. Am. J. Psychiatry 172, 638–646 (2015).
Collishaw, S., Hammerton, G. & Mahedy, L. Mental health resilience in the adolescent off spring of parents with depression: a prospective longitudinal study. Lancet Psychiatry 1, 49–57 (2016).
PaillèreMartinot, M. et al. White-matter microstructure and gray-matter volumes in adolescents with subthreshold bipolar symptoms. Mol.Psychiatr. 19, 462–470 (2014).
Vulser, H. M. et al. Subthreshold depression and regional brain volumes in young community adolescents. J. Am. Acad. Child Adolesc. Psychiatry 54, 832–840 (2015).
Gasquoine, P. G. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 37, 340–389 (2013).
Luna, B., Marek, S., Larsen, B., Tervo-Clemmens, B. & Chahal, R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 38, 151–170 (2015).
Haber, S. N. & Behrens, T. E. J. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron 83, 1019–1039 (2014).
Botvinick, M. M., Cohen, J. D. & Carter, C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8, 539–546 (2004).
Beckmann, M., Johansen-Berg, H. & Rushworth, M. F. S. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190 (2009).
Duffy, A., Goodday, S., Keown-Stoneman, C. & Grof, P. The emergent course of bipolar disorder: observations over two decades from the canadian high-risk offspring cohort. Am. J. Psychiatry 176, 720–729 (2019).
Pannekoek, J. N. et al. Reduced anterior cingulate gray matter volume in treatment-naïve clinically depressed adolescents. NeuroImage Clin. 4, 336–342 (2014).
Drevets, W. C., Price, J. L. & Furey, M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118 (2008).
Yu, H. et al. Common and distinct patterns of grey matter alterations in borderline personality disorder and bipolar disorder: voxel-based meta-analysis. Br. J. Psychiatry 215, 395–403 (2019).
Kumano, H. et al. Brain metabolic changes associated with predispotion to onset of major depressive disorder and adjustment disorder in cancer patients -a preliminary PET study. J. Psychiatr. Res. 41, 591–599 (2007).
Lin, K. et al. Inflammation, brain structure and cognition interrelations among individuals with differential risks for bipolar disorder. Brain Behav. Immun. 83, 192–199 (2020).
Tao, J. et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. NeuroImage Clin. 23, 101834 (2019).
Suo, C. et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 11, 1633–1642 (2016).
Wegner, M. A. S. K. A. Systematic review of meta-analyses: excercise effects on depression in children and adolescents. Front. Psychiatry 11, 81 (2020).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (NSFC:81671347), Science and Technology Plan Project of Guangdong Province (2019B030316001), and the National Key R&D Program of China (2016YFC1306702).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is not any conflict of interest in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, K., Stubbs, B., Zou, W. et al. Aerobic exercise impacts the anterior cingulate cortex in adolescents with subthreshold mood syndromes: a randomized controlled trial study. Transl Psychiatry 10, 155 (2020). https://doi.org/10.1038/s41398-020-0840-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-0840-8
This article is cited by
-
Sport und körperliche Bewegung bei unipolarer Depression
Der Nervenarzt (2021)