Abstract
The aim of this review is to summarize evidence regarding rat emotional experiences during carbon dioxide (CO2) exposure. The studies reviewed show that CO2 exposure is aversive to rats, and that rats respond to CO2 exposure with active and passive defense behaviors. Plasma corticosterone and bradycardia increased in rats exposed to CO2. As with anxiogenic drugs, responses to CO2 are counteracted by the administration of anxiolytics, SRIs, and SSRI’s. Human studies reviewed indicate that, when inhaling CO2, humans experience feelings of anxiety fear and panic, and that administration of benzodiazepines, serotonin precursors, and SSRIs ameliorate these feelings. In vivo and in vitro rat studies reviewed show that brain regions, ion channels, and neurotransmitters involved in negative emotional responses are activated by hypercapnia and acidosis associated with CO2 exposure. On the basis of the behavioral, physiological, and neurobiological evidence reviewed, we conclude that CO2 elicits negative emotions in rats.
Similar content being viewed by others
Introduction
Several behavioral studies indicate that carbon dioxide (CO2) elicits negative responses and is aversive to rats1,2,3,4,5,6,7,8 although not all studies agree9,10. Rat exposure to CO2 is a well-accepted translational model for the understanding of fear, anxiety, dyspnea (feeling of breathlessness), and panic in humans11.
Pain, fear, panic, and anxiety are considered high arousal, negatively valenced emotional states. Here, we follow a functional working definition that identifies emotional responses as objectively observable, and feelings of emotions as the conscious awareness of emotions experienced as positive or negative12,13. Emotions are “central states” inferred from brain arousal, and behavioral and physiological changes due to the presentation of a competent situation-dependent stimuli12. Not all stimuli elicit an emotional response; the competence of the stimuli will depend on the individual’s evolutionary history (innate response), personal experience (developmental plasticity and learning), discriminative properties of the stimuli (intensity and type of stimuli), and the current situation (e.g., controllability). Induction regions in the brain are responsible for the emotional cascade (chemical and neural reaction) that lead to the execution of appropriate behavioral, physiological, and brain responses to cope with a competent stimuli12.
Feelings of emotions can be described using a two-axis (arousal/valence) model14, and comprise different patterns of neural, behavioral, and physiological responses15. In the scientific literature, there is little consensus regarding what constitutes the feelings of emotions, and how and where these feelings are evoked in the brain. For example, some argue that this requires an internal self-representation of body and mind changes (i.e., interoception; homeostatic state, state of preparedness to cope, and motivational state) that accompany emotions, a process thought to occur in cortical areas of the brain (e.g., insula and cingulate cortex). Within this view, species that possess interoception could feel emotions12,15,16,17. Other authors emphasize the role of neocortical working memory (i.e., temporary hold and manipulation of information while doing mental work) as a requirement for feeling emotions, an idea that may exclude some nonhuman animals18. Panksepp13 argued that basic neurobiological subcortical areas present within all mammals are responsible for both emotion and feelings.
The assessment of felt emotions remains a major challenge13,19, but strong inferences about felt experiences can be made through a combination of evidence from (1) central state emotions (i.e., regional and local brain arousal, and behavioral and physiological changes when a competent situation-dependent stimuli is present)12,15,20, (2) indications of awareness (i.e., behavioral plasticity, direction and maintenance of attention, and agency)19, (3) functional homology in which human felt experiences and their associated physiological, neural, and behavioral responses can be cautiously compared to that of animal responses to the same stimuli and used as a proxy to the animal’s felt emotions13,21, and (4) drug treatments that target specific feelings of emotions in humans to infer specific feelings in animals19.
The aim of this paper is to review the available information on the behavioral, physiological, and neurological effects of CO2 on rat emotional responses, discussing inconsistencies between studies. We also cite research on human felt experiences when inhaling CO2 as context for understanding of rat emotional experiences. We focus on rats given the mounting research done in this species, but also refer to mouse studies when appropriate. This review includes only articles that were peer-reviewed, written in English, that assessed the effects of CO2 inhalation (or hypercapnia), and that report at least one outcome (behavioral, physiological, or neurobiological) indicative of an emotional response. Throughout the review, we describe the magnitude of effects only when studies reported these as statistically significant.
Biological responses
Inhaling high concentrations of CO2 suppresses the removal of metabolic waste CO2. The increase in CO2 metabolic waste results in high arterial PaCO2 (hypercapnia), decreasing blood pH (acidosis; pH < 7.2)22. Since acid–base and PaCO2 balance are important for survival, mammals possess mechanisms of detecting changes in pH and CO2, and responding to these threats to homeostasis. A rise in arterial PaCO2 and decrease in pH are detected by peripheral and central chemoreceptor cells. A synergic output from the integration of peripheral and central chemoreceptor inputs23, adjusts the ventilatory response according to the blood gas stimuli22. For example, when exposed to the 20% CO2 challenge (rapidly increasing concentration stabilizing at 20% CO2 after 5 min), rats showed increased breathing frequency from 100 to 130 breaths per minute (b.p.m.) within the first minute, and 130–165 b.p.m. after 2 min of exposure24. Similar physiological responses are found in humans; when subjected to inhalation of 5–7% CO2, healthy humans increase both tidal volume and respiratory frequency as a compensatory mechanism to remove excess CO225.
By modulating ventilation, mammals can cope with slight increases in CO2, but when increased ventilation is not sufficient to remove excess CO2 the animal may be experiencing negative emotional states, such as air hunger21, as evidenced by behavioral and physiological responses, and brain activation.
Behavioral responses
Here, we discuss the behavioral evidence of negative emotional states in three sections: (1) studies where animals cannot escape exposure to CO2, (2) studies where animals can avoid exposure to CO2, and (3) other situations where behavior is used to infer negative emotional states during or after exposure to CO2.
Inescapable exposure to CO2
To assess emotions evoked by CO2, one common approach is forced exposure to the gas6,9,26. The working (and typically implicit) assumption is that the frequency, duration, and intensity of the response reflects the intensity of the rat’s negative emotional experience to the procedure.
Several studies have found behavioral evidence of negative states in rats exposed to CO2. Niel and colleagues6,7, using ~17% CO2 chamber vol. min−1, found that rats showed increased frequencies and intensities of several behaviors associated with distress. The onset of rearing and increased locomotion occurred at ~5% CO2 and peaked at ~20% CO2. Escape behaviors (i.e., pushing and scratching at the lid) were observed at between 20 and 28% CO2. These results are consistent with others using slightly higher flow rates (18.5 and 23% CO2 chamber vol. min−1)27,28. Vocalizations in the range of 6–103 kHz have been reported for rats exposed to CO2 flow rates between 17 and 30% chamber vol. min−1 (refs. 2,6). However, one other study reported that rats exposed to 10% CO2 chamber vol. min−1 did not vocalize, and that locomotion and rearing did not increase relative to baseline levels29.
Rats exposed to the 20% CO2 challenge show variable results. Some studies have reported increases in locomotion but not freezing24, and others have found the reverse30. These results suggest that the type of defense behavior varies (between active and passive), but that some response is usually present.
When exposed to high concentrations of static CO2 (CO2 > 97%), some studies have reported that rats are less active and do not show struggling, vocalizations9, or other signs of distress31, but others have reported signs of asphyxia and behavioral excitation3. In these studies, however, behaviors were sometimes recorded without baseline or acclimation periods9, and behavioral responses were not clearly defined. For example, “head rising” was described as “inquisitive or agitated movements of head,” vocalizations as “squealing and other noises,” and escape as “attempts to get out of the box”31, or responses were simply mentioned without description3. Without control animals, baseline observations, and a clear description of behaviors, interpretation of these results is challenging.
Strain differences in rat responses to CO2 have been seldom assessed. Winter and colleagues32 showed that exposure to 10% static CO2 elicits freezing behavior in Long Evans and Wistar Kyoto strains, but not in Sprague Dawley and Wistar strains. Sprague Dawley rats often respond to CO2 exposure by increased active defense behavioral responses2,6,24,27,28,33, but the absence of responses has also been also reported for this strain29,31. In contrast, Lister Hooded rats decrease activity during CO2 exposure26. Blackshaw et al9. reported a decrease in activity by Wistar rats during exposure to CO2, a result that differs from the findings of Niel et al7. Fisher rats—a strain selected for lower activity—showed no behavioral signs of distress when exposed to CO2 gradual fill10. In comparison to Sprague Dawley, Brown Norway rats show more digging in response to CO2 exposure33. These results suggest that strain differences limit comparability among studies.
Most CO2 exposure studies have used male rats. Male rats responded with increased active defense in some studies using forced exposure to CO23,6,7,24,28,34, but other studies report no changes in behavior10,29,31. Female rats vocalized2 and increased active defense behaviors27 during CO2 forced exposure, but in another study showed no change in behavior9. Two studies recently published found no differences in behavioral responses to CO2 between male and female rats33,35.
Individual differences in rat responses to forced exposure to CO2 are often mentioned. It has been reported that only 20% of rats climbed the cage and 20% circled (i.e., moving around the perimeter of the cage)31, some rats expressed little and others numerous escape behaviors6, and only half of the rats tested increased locomotion7. Variation in rat response to CO2 may be related to reactivity36. For example, it has been shown that individual differences in active defense behaviors are consistent between two forced exposures to CO2 (ref. 27).
In summary, there is considerable variation in responses of rats to forced CO2 exposure within and between studies. Strain, sex, and individual differences account for some of this variability, but contrasting results within the same sex and strain still exist. Within-study variation in rat responses could be reflective of individual differences in CO2 reactivity. Differences in methodology, including the use of baselines, controls, type of cages, and induction method (e.g., static versus gradual fill, variable concentrations, and flows rates), and the lack of well-defined behaviors and interpretation of behaviors limit comparability between studies. Nonetheless, considering the responses summarized in Table 1, it seems likely that rats exposed to CO2 experience negative emotional states when escape is prevented.
Escapable exposure
Here, we describe the behavioral responses of rats when exposure to CO2 can be avoided. One approach to assess emotions during CO2 exposure is through choice and motivational tests. This approach is based upon the “hedonic principle;” i.e., that animals are motivated to avoid undesired end states (e.g., potential harms, pain, etc.) and approach desired ones37.
Choice tests involve giving animals two or more alternative conditions (e.g., different agents or the same agent at different concentrations), and measuring the amount of time spent in each alternative as an expression of preference4. Studies have shown that rats prefer lower than higher concentrations of CO2. Rat spent between 36 and 51 s in a chamber prefilled with room air, and 2.1 and 0.7 s in chambers with 25.5% and 50.8% CO2, respectively5.
The strength of aversion can be measured by giving rats the ability to avoid exposure, with an added cost to the avoidance38. For inhalant agents, strength of aversion has been investigated through aversion- and approach-avoidance tests. In the aversion-avoidance test, the cost of avoiding the agent in a preferred dark compartment is exposure to an aversive brightly lit compartment. Using the aversion-avoidance test with a flow rate of 24% chamber vol. min−1, all rats left the dark chamber filling with CO2, escaping to the previously avoided bright chamber39.
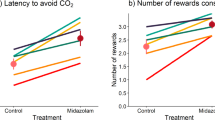
In the approach-avoidance test, the cost of escaping to an agent free cage is the loss of a sweet reward. Rats are highly motivated to eat sweet rewards even when fed their regular diet at libitum40. When tested with different static concentrations of CO2 in the approach-avoidance apparatus, rats tolerated concentrations ranging from <1 to 10% CO2, entering the test chamber, eating the sweet rewards, and staying in the gas chamber for ~300 s. However, at 10% CO2 rats stopped eating ~20 s earlier than when tested with 5% CO2, indicating that although 10% CO2 is aversive, rats will tolerate exposure to obtain the sweet rewards. But the latency to leave the gas chamber decreased to 46 s at 15% CO2, and to 5 s with 20% CO2 (ref. 41).
Other studies using flow rates between 14 and 19% CO2 chamber vol. min−1, have shown that aversion is variable. In the aversion-avoidance test, latency to avoid CO2 ranged from 7 to 48 s between rats39. In the approach-avoidance test, the concentration avoided varied among individuals ranging from 11 to 18.6% CO2 (ref. 7). Variation among individuals in rat aversion to CO2 has shown to be consistent across exposures, within aversion27- and approach-avoidance27,42. These results support the idea that rats vary in CO2 reactivity. However, all aversion studies report that rats avoid CO2 before becoming ataxic or recumbent, even when fasted for 24 h (ref. 40).
These results (summarized in Table 2) indicate that onset of negative emotional states occurs ~10% CO2, although some rats are willing to tolerate exposure up to 18% CO2. These concentrations are consistent with the onset and peak of active behaviors during forced exposure6, and freezing behaviors reported using the 20% CO2 challenge30.
Human feelings of immobility (freezing), feeling paralyzed, desire to flee, and wanting to leave the room following inhalation of similar concentrations of CO2 (20–35% CO2)43,44, seem consistent with the rat defense behaviors reported above. Humans report feelings of anxiety at lower CO2 concentrations, and fear and panic at higher concentrations. For example, prolonged inhalation (20 min) of low CO2 concentrations (7.5%) elicits anxiety and induces hypervigilance in healthy volunteers45. This emotional experience is likely related to hypercapnia (rather than to hypoxia), since CO2 was administered to provide a normoxic gas mixture (containing ~21% O2)46. Increasing doses (e.g., double inhalation of 35% CO2), often induce panic attacks in healthy people47. These results provide some basis for suggesting that rat behavioral responses to CO2 may also be associated with the onset of negative emotional states related to feelings of anxiety, fear, or panic.
As reviewed above, the behavioral responses of rats vary between individuals. This variation has also been reported for human subjects. Human sensitivity to CO2 falls on a continuum panic disorder (PD) patients being the most sensitive48. In healthy humans, increases in fear and anxiety were reported in only 50% of the participants after a double inhalation of CO2 concentrations between 9 and 35% (ref. 49). When healthy humans inhale 20% CO2 for 20 s, 13% and 20% of the individuals experience modest or greater feelings of immobility and desire to flee, respectively44. With a double inhalation of 35% CO2, 47–68% healthy humans experience panic attacks47. In PD patients, a single inhalation of 35% CO2 induces panic in 43–94% of the individuals, depending on the subtype of PD48. These parallels in the human and rat literature are consistent with the idea that individual variation in rat behavioral responses to CO2 are associated with differences in the emotions elicited by this agent.
Exposure in other situations
CO2 is often used as an unconditioned stimulus in studies designed to induce negative emotional states in rodents. These studies support the conclusion that CO2 exposure is anxiogenic. In the Vogel test, two opposing motivations—gaining a reward versus avoiding a punishment—are used to assess the anxiolytic and anxiogenic effects of drugs. Food or water-deprived rats can choose to receive a reward (water or food) at the cost of receiving punishments (shocks); anxiogenic drugs suppress reward consumption and anxiolytics increase reward consumption50. Using the Vogel test, rats previously exposed for 60 s to CO2:O2 (35:65%) showed a similar response to that of rats treated with the anxiogenic benzodiazepine receptor ligand FG 7142; rats exposed to CO2 suppressed water licking by 40% relative to control rats51, indicating that CO2 exposure has an anxiogenic effect.
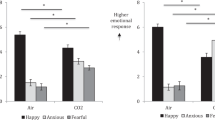
In the open field test, the tendency to avoid the central area and display thigmotaxis (locomotion close to the walls of the apparatus) is enhanced by anxiogenic drugs, while anxiolytics increase locomotion in the central areas of the arena52. In the social interaction test, anxiogenic drugs decrease the frequency of social interactions (e.g., sniffing, following, and grooming) while anxiolytics have the opposite effect53. After exposure to the 20% CO2 challenge, rats showed a 15% increase in thigmotaxis34 and a 50% decline in social interactions compared to rats exposed to air24.
Conditioning tests are also used to assess the aversiveness of a stimulus, and different variants of this test can be found. Potentially aversive stimuli can be paired with a neutral stimulus (Pavlovian conditioning), such as a neutral environment (place conditioning). Rats are then later exposed only to the paired stimulus, in absence of the aversive stimulus. Avoidance of the stimulus is an indicator of aversion, but if avoidance is restricted then immobility can be used as a measure of aversion54. Rats exposed to vanilla scent before 30 s of forced inhalation of different concentrations of CO2 (<1, 5, 35, or 100%), showed a conditioned response to vanilla 24 h later. Rats that inhaled <1% CO2 froze less than rats that inhaled higher concentrations. Rats exposed to 100% CO2 froze more and this conditioning resisted extinction relative to rats exposed to 5% CO2. These results indicate that CO2 can be used to condition anxiety in rats, and that the degree of behavioral response (freezing) and extinction reflect the severity of the experience55.
In summary, rat emotional states induced by CO2 inhalation are sustained after exposure, and CO2 acts as an unconditioned negative stimulus especially at higher concentrations (Table 3).
Physiological responses
Sympathetic responses in rats exposed to CO2 include increased blood pressure31 and bradycardia before the loss of righting reflex (LORR)1. In rats, arterial blood pressure increases during the 20% CO2 challenge24. Bradycardia has been reported for rats exposed to flow rates between 10 and 20% CO2 chamber vol. min−1 (refs. 1,29), and to the 20% CO2 challenge24. The cardiovascular response also correlates with changes in PaCO2 and pH (ref. 34). Altholtz et al.56 found that rats anesthetized with 70% CO2 showed increased plasma corticosterone levels after 30 min (but see ref. 35); similar results were found for rats exposed to 35% CO2 (ref. 57). In humans, inhalation of CO2 concentrations between 7 and 14% for 10–20 min induces an increase in minute ventilation, blood pressure, heart rate, plasma noradrenaline, and cortisol58. A single inhalation of 35% CO2 activates the HPA axis, and cortisol increases for ~30 min after exposure. In addition, blood pressure increases and heart rate decreases with exposure43,59.
The sympathetic and neuroendocrine responses to CO2 exposure indicate arousal likely reflective of negative valence. When taken together with the behavioral responses described in the previous section, we suggest that these physiological responses to CO2 exposure are associated with negative emotional states.
Neurobiological responses
In this section, we review the effects of CO2 on activation of brain regions, and nuclei within regions, involved in fear and anxiety. Chemoreceptive areas of the brain implicated in the ventilatory response to hypercapnia (e.g., medullary raphe, retrotrapezoid nucleus, caudal medulla, nucleus tractus solitaries, etc.) have been previously reviewed60,61.
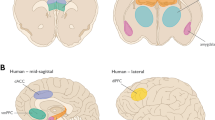
The amygdala is implicated in emotional responses to sensory inputs, and in generating the behavioral and physiological adaptive responses62. In rats exposed to 10% CO2, the medial and central amygdala show increased c-Fos expression. This increase is associated with augmented minute ventilation, breathing frequency, and tidal volume63. Johnson et al.64 found that rats exposed to the 20% CO2 challenge tended to increase c-Fos expression in the central amygdala, and this increase was related to increased fecal boli production (indicative of fear and anxiety) and thigmotaxis in the open field tests, but no effect of hypercapnia on c-Fos expression was detected in the medial and basolateral amygdala. Increased c-Fos expression in the central and basolateral amygdala has been found in high CO2-sensitive mice (i.e., mice that show a higher freezing responses to 5% CO2 than low responders) due to CO2 inhalation compared to that of mice exposed to air65. Asic1a+/+ mice (mice with intact acid sensing ion channels 1a in the amygdala and in other brain regions) froze when exposed to 10% static CO2, increased thigmotaxis when exposed to 20% CO2 in an open field test and preferred an air chamber, to a chamber prefilled with 20% CO2 in a choice test. These responses were attenuated in Asic1a−/− mice (mice without intact acid sensing ion channels). In vitro amygdala neurons of Asic1a+/+, but not Asic1a−/− mice, responded to a reduction in pH. In addition, CO2 inhalation decreased pH in the basolateral amygdala of Asic1a+/+ mice. Focal acidification of this region elicited a strong freezing response, while the administration of HCO3− (to counteract acidosis) reduced freezing due to CO2 inhalation66. Overall, these results suggest that the amygdala acts as a chemoreceptor for changes in PaCO2/H+, and its activation is involved in the behavioral response to CO2.
The bed nucleus of the stria terminalis (BNST), frequently referred as the extended amygdala, is associated with modulation of behavioral responses to threatening stimuli67. Taugher and colleagues68 have shown that the BNST is also involved in the behavioral response to hypercapnia. These authors found that lesions in the BNST decreased freezing responses of mice during 10% CO2 exposure. When compared air exposure, inhalation of 5% CO2 increased c-Fos expression in the BNST of high CO2-sensitive mice65. In rats, c-Fos expression in the BNST did not differ when exposed to the 20% CO2 challenge versus air64.
Activation of the hypothalamus is related to the execution of different behavioral and physiological responses; the most commonly identified is the activation of the HPA axis and modulation of autonomic responses. During the HPA axis cascade, the paraventricular nucleus (PVN) in the hypothalamus secretes corticotropin-releasing factor69. Several studies indicate that when rats are exposed to CO2 concentrations of between 5 and 20%, the PVN shows a high density of positive c-Fos expression, indicating PVN activation during hypercapnia64,70,71,72. One other study reports no significant increase in c-Fos expression in the PVN of rats exposed to 10% CO2 (ref. 63).
The dorsomedial region of hypothalamus (DMH) is involved in the execution of behavior responses to aversive stimuli73,74. One study reported that rats exposed to 10% CO2 did not differ from controls in the number of labeled cells on the DMH63. However, other studies have found that the DMH shows high c-Fos expression in rats exposed to 5% CO2 and the 20% CO2 challenge30,64,70, particularly in orexin neurons34 found only in the DMH and perifornical nucleus of the hypothalamus75. Thigmotaxis in the open field was attenuated in rats treated with an orexin receptor antagonist before the 20% CO2 challenge34. Orexin neurons are chemosensitive; the firing rate of in vitro orexin neurons increases with fluctuations in CO2 and pH (ref. 76). Furthermore, individual differences in rat behavioral responses to the CO2 challenge were related to variation in orexin activity in the lateral hypothalamus36. These results show that the DMH and the perifornical nucleus of the hypothalamus are involved in the behavioral response to hypercapnia, and act as chemoreceptors.
Another region relevant in the modulation of behavioral responses to threatening stimuli is the periaqueductal gray (PAG). The ventrolateral PAG (VLPAG) is involved in immobility responses77, while the dorsal PAG (dorsolateral DLPAG and dorsomedial DMPAG) is related to flight behaviors78,79. The VL, DL, and DMPAG of rats exposed to CO2 show a dose-dependent increased c-Fos expression75,80. Rats exposed to CO2 concentrations between 8 and 13% show increased immobility and flight behaviors associated with PAG electrical stimulation81. Lesions in the DL and DMPAG of rats exposed to low concentrations of CO2 (7% CO2) decreased the ventilatory response compared to controls, without altering the cardiovascular response82. These results indicate that the PAG is activated during CO2 inhalation and involved in the behavioral and ventilatory responses to hypercapnia.
Consistent with this literature on rats (summarized in Table 4), research on human subjects shows that CO2 inhalation is associated with the activation of the amygdala, PAG, hypothalamus, and anterior insula. Moreover, this work on humans shows that activation of these brain regions is correlated with feelings of dyspnea83. Interestingly, patients with bilateral amygdala lesions still experienced fear and panic when inhaling 35% CO2 (ref. 84), but not when exposed to external life-threatening stimuli85.
A key central chemoreceptor region is the medullary raphe; local acidification of the medullary raphe produces an increase in the ventilatory response86. Serotonin (5HT) is originated in the medullary raphe by tryptophan hydroxylation. Serotonin is implicated in mediating emotional states, perception, cognition, and sympathetic arousal87. Administration of serotonin reuptake inhibitors (SRIs), which increase synaptic serotonin, decreases anxiety in humans, and reduces respiratory rate of rats exposed to 6% CO2 (ref. 88). Mice treated with selective serotonin reuptake inhibitors (SSRIs) showed a decrease in freezing responses and ventilatory response during 5% CO2 exposure, and showed less context conditioning (rearing behavior) after exposure, than did control mice89. These results indicate that serotonin is involved in the ventilatory and behavioral response to hypercapnia. In humans, treatment with serotonin antagonists, or tryptophan depletion before one or two inhalations of 35% CO2, enhances the experience of anxiety, fear, dyspnea, and panic90,91. Treatment with serotonin precursors and SSRIs have the opposite effect92,93.
The role of the γ-aminobutyric acid (GABA)—a mammalian neurotransmitter that mediates synaptic inhibitions and is associated with anxiety94—is also involved in the response to hypercapnia. In rats, stressful events like acute handling95, chronic restraint96, and social isolation97 decrease GABAA receptor function. The administration of compounds that bind to benzodiazepine recognition sites and that decrease the function of GABAA receptors (i.e., anxiogenic drugs) induce anxiety-like behavior in rats98. Likewise, exposure to 35% CO2 decreases GABAA function in the rat cerebral cortex, cerebellum, and hippocampus. The administration of benzodiazepines—which bind to the benzodiazepine receptor sites enhancing GABAergic transmission—before exposure to CO2, counteracts the effects of CO2 on GABAA receptor functioning99,100, increase reward consumption in a Vogel conflict test51, increase tolerance to CO2 in approach-avoidance42, and enhance social interactions in the social test (at doses that do not impair locomotor activity)75. These results indicate that CO2 acts as negative stimulus on GABAA functioning, and that following drug treatment with known anxiolytics the behavioral effect of CO2 is diminished. Similarly, healthy people pretreated with alprazolam before 5, 7.5, and 35% CO2 inhalation, show diminished experiences of fear, feeling like leaving the room, dyspnea, panic, and distress101,102.
In summary, brain areas, ion channels, and neurotransmitters involved in fear and anxiety are activated by hypercapnia. These regions include the amygdala, BNST, hypothalamus, and PAG. This body of evidence is consistent with the conclusion that rats experience negative emotions when inhaling CO2. Since these responses can, to some extent, be counteracted by the administration of anxiolytics and SRIs, and similarly to behavioral changes in rats, benzodiazepines, serotonin precursors, and SRIs ameliorate feelings of anxiety, fear, distress, dyspnea, and panic due to hypercapnia in humans.
Conclusions
Concentrations <10% CO2 are tolerated, do not elicit intense behavioral responses, and cause mild conditioning in rats. Rats respond with active and passive defense behaviors to concentrations >10%. If escape is possible all rats avoid CO2, even to concentrations <10%, but when motivated some rats may tolerate higher concentrations (up to 18% CO2). The behavioral responses in the open field and social tests show that negative emotions, which resemble those associated with exposure to anxiogenic drugs, were sustained when CO2 was no longer present. Exposure to concentrations over 35% has anxiogenic effects and produces strong conditioning. The level of conditioning and extinction resistance depend upon CO2 concentration, implying that the magnitude of the emotional response increased with the intensity of the stimuli. The behavioral responses to CO2 are accompanied with neuroendocrine and sympathetic activation. Brain responses related to fear and anxiety are detected when rats inhale CO2. Overall, these studies indicate that CO2 elicit negative emotional states in rats.
Humans report feelings of fear, anxiety, dyspnea, distress, and panic during CO2 inhalation, and the evidence reviewed above suggests that rats experience similar emotions. Caution is required when functional homology is used to draw inferences regarding felt experiences19, but ventilatory and cardiovascular changes due to CO2 inhalation are similar between rats and humans, behavioral responses of rats when exposed to CO2 correspond well with feelings reported by humans following CO2 inhalation, and human feelings of anxiety, fear, dyspnea, and panic in response to hypercapnia can be attenuated by benzodiazepines and SSRIs (drugs that also diminish the defense behaviors seen in rats exposed to CO2). In addition, variation in rat and human CO2 reactivity is linked to the emotional experience during inhalation.
Throughout this review, we used functional homology to link rat and human felt emotions during CO2 inhalation. Although such inferences require caution, there is considerable research in human felt emotions due to CO2 inhalation. One example of a study comparing rodents and humans using the same CO2 concentrations is that by Leibold and colleagues103. These authors used static 9% CO2 concentrations to compare behavioral, respiratory, and cardiovascular responses of mice and humans. This study found that mice avoided the central areas of an open field, showed immobility responses and produced fecal boli during 9% CO2 exposure, and humans reported an increase in feelings of fear and panic due to 9% CO2 inhalation. Inhalation of 9% CO2 induced bradycardia in both species. The authors concluded that mice reactivity to CO2 can be used as model for humans. We suggest also that the opposite is also true; i.e., that human reports can be used to better understand the emotional experience of rodents during CO2 exposure.
Taken together, this evidence supports our conclusion that rats experience negative emotions during and after CO2 exposure, and that these emotions likely correspond to fear, anxiety, dyspnea, distress, and panic. This work contributes to the study of translational models of anxiety and panic, and is also relevant to ongoing debate regarding the use CO2 for killing animals. In addition, we suggest that this literature will be of interest to all who study felt emotions in animals.
References
Chisholm, J. M. & Pang, D. S. Assessment of carbon dioxide, carbon dioxide/oxygen, isoflurane and pentobarbital killing methods in adult female Sprague-Dawley rats. PLoS ONE 11, e0162639 (2016).
Chisholm, J., De Rantere, D., Fernandez, N. J., Krajacic, A. & Pang, D. S. Carbon dioxide, but not isoflurane, elicits ultrasonic vocalizations in female rats. Lab. Anim. 47, 324–327 (2013).
Coenen, A. M. L., Drinkenburg, W. H. I. M., Hoenderken, R. & Van Luijtelaar, E. L. J. M. Carbon dioxide euthanasia in rats: oxygen supplementation minimizes signs of agitation and asphyxia. Lab. Anim. 29, 262–268 (1995).
Leach, M. C., Bowell, V. A., Allan, T. F. & Morton, D. B. Aversion to gaseous euthanasia agents in rats and mice. Comp. Med. 52, 249–257 (2002).
Leach, M. C., Bowell, V. A., Allan, T. F. & Morton, D. B. Measurement of aversion to determine humane methods of anaesthesia and euthanasia. Anim. Welf. 13, S77–S86 (2004).
Niel, L. & Weary, D. M. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl. Anim. Behav. Sci. 100, 295–308 (2006).
Niel, L., Kirkden, R. D. & Weary, D. M. Effects of novelty on rats’ responses to CO2 exposure. Appl. Anim. Behav. Sci. 111, 183–194 (2008).
Niel, L., Stewart, S. A. & Weary, D. M. Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl. Anim. Behav. Sci. 109, 77–84 (2008).
Blackshaw, J. K., Fenwick, D. C., Beattie, A. W. & Allan, D. J. The behavior of chickens, mice and rats during euthanasia with chloroform, carbon dioxide and ether. Lab. Anim. 22, 67–75 (1988).
Hackbarth, H., Küppers, N. & Bohnet, W. Euthanasia of rats with carbon dioxide-animal welfare aspects. Lab. Anim. 34, 91–96 (2000).
Quagliato, L. A., Freire, R. C. & Nardi, A. E. The role of acid-sensitive ion channels in panic disorder: a systematic review of animal studies and meta-analysis of human studies. Transl. Psychiatry 8, 185 (2018).
Damasio, A. R. Emotions and Feelings (eds Manstead, A. S., Frijda, N. & Fischer, A.) (Cambridge University Press, 2004).
Panksepp, J. The Affective Brain and Core Consciousness: How does Neural Activity Generate Emotional Feelings? (eds Lewis, M., Haviland-Jones, J. M. & Barrett, L. F.) (Guilford Press 2008).
Mendl, M., Burman, O. H. & Paul, E. S. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B Biol. Sci. 277, 2895–2904 (2010).
Adolphs, R. The biology of fear. Curr. Biol. 23, R79–R93 (2013).
Adolphs, R. Emotion. Curr. Biol. 20, R549–R552 (2010).
Craig, A. D. Interoception and Emotion: a Neuroanatomical Perspective (eds Lewis, M., Haviland-Jones, J. M. & Barrett, L. F.) (Guilford Press 2008).
LeDoux, J. E. & Pine, D. S. Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry 173, 1083–1093 (2016).
Weary, D. M., Droege, P. & Braithwaite, V. A. Evidence of felt emotions: approaches, inferences, and refinements. Adv. Stud. Behav. 49, 27–48 (2017).
Mellor, D. Updating animal welfare thinking: moving beyond the “Five Freedoms” towards “a Life Worth Living”. Animals 6, 21 (2016).
Beausoleil, N. J. & Mellor, D. J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 63, 44–51 (2015).
Prange, H. Respiratory physiology: understanding gas exchange (Springer Science & Business Media 2012).
Blain, G. M., Smith, C. A., Henderson, K. S. & Dempsey, J. A. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J. Physiol. 588, 2455–2471 (2010).
Hickman, D. L. et al. Evaluation of low versus high volume per minute displacement CO2 methods of euthanasia in the induction and duration of panic-associated behavior and physiology. Animals 6, 45 (2016).
Gorman, J. M. et al. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: evidence for a central fear mechanism. Arch. Gen. Psychiatry 58, 125–131 (2001).
Britt, D. P. The Humaneness of Carbon Dioxide as an Agent of Euthanasia for Laboratory Rodents, 19–31 (Potter’s Bar, UK, 1987).
Améndola, L. & Weary, D. M. Evidence for consistent individual differences in rat sensitivity to carbon dioxide. PloS ONE 14, e0215808 (2019).
Makowska, I. J. & Weary, D. M. Using rat behavior to assess aversion to euthanasia agents. ALTEX 1, 465–467 (2012).
Burkholder, T. H. et al. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J. Am. Assoc. Lab. Anim. Sci. 49, 448–453 (2010).
Johnson, P. L., Hollis, J. H., Moratalla, R., Lightman, S. L. & Lowry, C. A. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J. Psychopharmacol. 19, 327–341 (2005).
Smith, W. & Harrap, S. B. Behavioral and cardiovascular responses of rats to euthanasia using carbon dioxide gas. Lab. Anim. 31, 337–346 (1997).
Winter, A., Ahlbrand, R., Naik, D. & Sah, R. Differential behavioral sensitivity to carbon dioxide (CO2) inhalation in rats. Neuroscience 346, 423–433 (2017).
Hickman, D. L. Home cage compared with induction chamber for euthanasia of laboratory rats. J. Am. Assoc. Lab. Anim. Sci. 57, 729–733 (2018).
Johnson, P. L. et al. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology 37, 1911–1922 (2012).
Hickman, D. L. Wellbeing of alcohol-preferring rats euthanized with carbon dioxide at very low and low volume displacement rates. J. Am. Assoc. Lab. Anim. Sci. 58, 78–82 (2019).
Monfils, M. H. et al. Predicting extinction phenotype to optimize fear reduction. Psychopharmacol 236, 99–110 (2019).
Fraser, D. & Duncan, I. J. ‘Pleasures’, ‘pains’ and animal welfare: toward a natural history of affect. Anim. Welf. 7, 383–396 (1998).
Kirkden, R. D. & Pajor, E. A. Using preference, motivation and aversion tests to ask scientific questions about animals’ feelings. Appl. Anim. Behav. Sci. 100, 29–47 (2006).
Wong, D., Makowska, I. J. & Weary, D. M. Rat aversion to isoflurane versus carbon dioxide. Biol. Lett. 9, 20121000 (2013).
Kirkden, R. D. et al. The validity of using an approach-avoidance test to measure the strength of aversion to carbon dioxide in rats. Appl. Anim. Behav. Sci. 114, 216–234 (2008).
Niel, L. & Weary, D. M. Rats avoid exposure to carbon dioxide and argon. Appl. Anim. Behav. Sci. 107, 100–109 (2007).
Améndola, L., Ratuski, A. & Weary, D. M. Variation in the onset of CO2-induced anxiety in female Sprague Dawley rats. Sci. Rep. 9, 19007 (2019).
Argyropoulos, SpiliosV. et al. Inhalation of 35% CO2 results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinology 27, 715–729 (2002).
Schmidt, N. B., Richey, J. A., Zvolensky, M. J. & Maner, J. K. Exploring human freeze responses to a threat stressor. J. Behav. Ther. Exp. Psychiatry 39, 292–304 (2008).
Garner, M., Attwood, A., Baldwin, D. S. & Munafò, M. R. Inhalation of 7.5% carbon dioxide increases alerting and orienting attention network function. J. Psychopharmacol. 223, 67–73 (2012).
Garner, M., Attwood, A., Baldwin, D. S., James, A. & Munafò, M. R. Inhalation of 7.5% carbon dioxide increases threat processing in humans. Neuropsychopharmacology 36, 1557 (2011).
Leibold, N. K. et al. Carbon dioxide inhalation as a human experimental model of panic: the relationship between emotions and cardiovascular physiology. Biol. Psychol. 94, 331–340 (2013).
Colasanti, A., Esquivel, G., J Schruers, K., Griez, J. & On, E. the psychotropic effects of carbon dioxide. Curr. Pharm. Des. 18, 5627–5637 (2012).
Griez, E. J., Colasanti, A., Van Diest, R., Salamon, E. & Schruers, K. Carbon dioxide inhalation induces dose-dependent and age-related negative affectivity. PLoS ONE 2, e987 (2007).
Millan, M. J. & Brocco, M. The Vogel conflict test: procedural aspects, γ-aminobutyric acid, glutamate and monoamines. Eur. J. Pharmacol. 463, 67–96 (2003).
Cuccheddu, T. et al. Proconflict effect of carbon dioxide inhalation in rats. Life. Sci. 56, PL321–PL324 (1995).
Prut, L. & Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33 (2003).
File, S. E. & Seth, P. A review of 25 years of the social interaction test. Eur. J. Pharm. 463, 35–53 (2003).
Carlezon, W. A. in Opioid Research: Methods and Protocols (ed Pan, Z. Z.) (Humana Press Inc 2003).
Mongeluzi, D. L., Rosellini, R. A., Ley, R., Caldarone, B. J. & Stock, H. S. The conditioning of dyspneic suffocation fear: effects of carbon dioxide concentration on behavioral freezing and analgesia. Behav. Modif. 27, 620–636 (2003).
Altholtz, L. Y., Fowler, K. A., Badura, L. L. & Kovacs, M. S. Comparison of the stress response in rats to repeated isoflurane or CO2: O2 anesthesia used for restraint during serial blood collection via the jugular vein. J. Am. Assoc. Lab. Anim. Sci. 45, 17–22 (2006).
Barbaccia, M. L. et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology 63, 166–172 (1996).
Sechzer, P. H. et al. Effect of CO2 inhalation on arterial pressure, ECG and plasma catecholamines and 17-OH corticosteroids in normal man. J. Appl. Physiol. 15, 454–458 (1960).
Kaye, J. et al. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J. Neuroendocrinol. 16, 256–264 (2004).
Guyenet, P. G. & Bayliss, D. A. Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961 (2015).
Nattie, E. & Li, A. Central chemoreceptors: locations and functions. Compr. Physiol. 2, 221–254 (2012).
Davis, M. & Whalen, P. J. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13 (2001).
Tenorio‐Lopes, L. et al. Neonatal maternal separation opposes the facilitatory effect of castration on the respiratory response to hypercapnia of the adult male rat: evidence for the involvement of the medial amygdala. J. Neuroendocrinol. 29, e12550 (2017).
Johnson, P. L. et al. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J. Psychopharmacol. 25, 26–36 (2011).
McMurray, K. M., Gray, A., Horn, P. & Sah, R. High behavioral sensitivity to carbon dioxide associates with enhanced fear memory and altered forebrain neuronal activation. Neuroscience 429, 92–105 (2020).
Ziemann, A. E. et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139, 1012–1021 (2009).
Davis, M. & Shi, C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann. N. Y. Acad. Sci. 877, 281–291 (1999).
Taugher, R. J. et al. The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. J. Neurosc. 34, 10247–10255 (2014).
Swanson, L. W. & Sawchenko, P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 6, 269–324 (1983).
Berquin, P., Bodineau, L., Gros, F. & Larnicol, N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 857, 30–40 (2000).
Haxhiu, M. A., Yung, K., Erokwu, B. & Cherniack, N. S. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Resp. Physiol. 105, 35–45 (1996).
Kc, P. et al. CO2-induced c-Fos expression in hypothalamic vasopressin containing neurons. Resp. Physiol. 129, 289–296 (2002).
Shekhar, A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 627, 9–16 (1993).
Shekhar, A. & DiMicco, J. A. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology 26, 407–417 (1987).
Johnson, P. L. et al. Orexin 1 and 2 receptor involvement in CO2-induced panic-associated behavior and autonomic responses. Depress Anxiety 32, 671–683 (2015).
Williams, R. H., Jensen, L. T., Verkhratsky, A., Fugger, L. & Burdakov, D. Control of hypothalamic orexin neurons by acid and CO2. Proc. Natl Acad. Sci. USA 104, 10685–10690 (2007).
Bago, M. & Dean, C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT 1A receptors in the RVLM. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R976–R984 (2001).
Beckett, S. & Marsden, C. A. The effect of central and systemic injection of the 5-HT1A receptor agonist 8-OHDPAT and the 5-HT1A receptor antagonist WAY100635 on periaqueductal grey-induced defence behavior. J. Psychopharmacol. 11, 35–40 (1997).
Bandler, R. & Shipley, M. T. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosc. 17, 379–389 (1994).
Teppema, L. J. et al. Expression of c‐fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J. Com. Neurol. 388, 169–190 (1997).
Schimitel, F. G. et al. Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat. Neuroscience 200, 59–73 (2012).
Lopes, L. T., Patrone, L. G., Bícego, K. C., Coimbra, N. C. & Gargaglioni, L. H. Periaqueductal gray matter modulates the hypercapnic ventilatory response. Pflug. Arch. Eur. J. Phy. 464, 155–166 (2012).
Liotti, M. et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc. Natl Acad. Sci. USA 98, 2035–2040 (2001).
Feinstein, J. S. et al. Fear and panic in humans with bilateral amygdala damage. Nat. Neurosci. 16, 270–272 (2013).
Feinstein, J. S., Adolphs, R., Damasio, A. & Tranel, D. The human amygdala and the induction and experience of fear. Curr. Biol. 21, 34–38 (2011).
Richerson, G. B. Serotonergic neurons as carbon dioxide sensors that maintain homeostasis. Nat. Rev. Neurosci. 5, 449–461 (2004).
Lesch, K. P., Araragi, N., Waider, J., van den Hove, D. & Gutknecht, L. Targeting brain serotonin synthesis: insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behavior. Philos. Trans. R. Soc. Lond. B 367, 2426–2443 (2012).
Olsson, M., Annerbrink, K., Bengtsson, F., Hedner, J. & Eriksson, E. Paroxetine influences respiration in rats: implications for the treatment of panic disorder. Eur. Neuropsychopharmacol. 14, 29–37 (2004).
McMurray, K. M., Strawn, J. R. & Sah, R. Fluoxetine modulates spontaneous and conditioned behaviors to carbon dioxide (CO2) inhalation and alters forebrain-midbrain neuronal activation. Neuroscience 396, 108–118 (2019).
Ben-Zion, I. Z., Meiri, G., Greenberg, B. D., Murphy, D. L. & Benjamin, J. Enhancement of CO2-induced anxiety in healthy volunteers with the serotonin antagonist metergoline. Am. J. Psychiatry 156, 1635–1637 (1999).
Klaassen, T., Klumperbeek, J., Deutz, N. E., van Praag, H. M. & Griez, E. Effects of tryptophan depletion on anxiety and on panic provoked by carbon dioxide challenge. Psychiatry Res. 77, 167–174 (1998).
Perna, G. et al. Antipanic drug modulation of 35% CO2 hyperreactivity and short-term treatment outcome. J. Clin. Psychopharmacol. 22, 300–308 (2002).
Schruers, K., van Diest, R., Overbeek, T. & Griez, E. Acute L-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatry Res. 113, 237–243 (2002).
Kaila, K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 42, 489–537 (2004).
Andrews, N., Zharkovsky, A. & File, S. E. Acute handling stress downregulates benzodiazepine receptors: reversal by diazepam. Eur. J. Pharmacol. 210, 247–251 (1992).
Gruen, R. J., Wenberg, K., Elahi, R. & Friedhoff, A. J. Alterations in GABA A receptor binding in the prefrontal cortex following exposure to chronic stress. Brain. Res. 684, 112–114 (1995).
Serra, M. et al. Social isolation induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J. Neurochem. 75, 732–740 (2000).
Biggio, G. et al. GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol. Therapeut. 48, 21–42 (1990).
Concas, A., Sanna, E., Cuccheddu, T. & Paola, M. Carbon dioxide inhalation, stress and anxiogenic drugs reduce the function of GABA A receptor complex in the rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 17, 651–661 (1993).
Sanna, E., Cuccheddu, T., Serra, M., Concas, A. & Biggio, G. Carbon dioxide inhalation reduces the function of GABAA receptors in the rat brain. Eur. J. Pharmacol. 216, 457–458 (1992).
Bailey, J. E., Papadopoulos, A., Seddon, K. & Nutt, D. J. A comparison of the effects of a subtype selective and non-selective benzodiazepine receptor agonist in two CO2 models of experimental human anxiety. J. Psychopharmacol. 23, 117–122 (2009).
Woods, S. W. et al. Carbon dioxide sensitivity in panic anxiety: ventilatory and anxiogenic response to carbon dioxide in healthy subjects and patients with panic anxiety before and after alprazolam treatment. Arch. Gen. Psychiatry 43, 900–909 (1986).
Leibold, N. K. et al. CO2 exposure as translational cross-species experimental model for panic. Transl. Psychiatry 6, e885 (2016).
Acknowledgements
We thank Catherine Schuppli, Marina Von Keyserlingk, Becca Franks, Huw Golledge, and anonymous referees for their helpful comments and suggestions. This research was funded by an NSERC Discovery grant to D.M.W. L.A. was supported by the CONACyT PhD. scholarship (no. 381124), the James A. Shelford Memorial Scholarship, and the Charles River Scholarship in Animal Welfare.
Author information
Authors and Affiliations
Contributions
Contributed to the writing of the manuscript (L.A. and D.W.).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Améndola, L., Weary, D.M. Understanding rat emotional responses to CO2. Transl Psychiatry 10, 253 (2020). https://doi.org/10.1038/s41398-020-00936-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-00936-w
This article is cited by
-
The perspectives of UK personnel towards current killing practices for laboratory rodents
Scientific Reports (2023)