Abstract
Expectancy of physical and social pleasure (PSP) promotes excessive drinking despite the potential aversive effects of misuse, suggesting an imbalance in the response to reward and pain in alcohol seeking. Here, we investigated the competing roles of the reward and pain circuits in PSP expectancy and problem drinking in humans. Using fMRI data during resting (n = 180) and during alcohol cue exposure (n = 71), we examined the antagonistic effects of the reward-related medial orbitofrontal cortex (mOFC) and pain-related periaqueductal gray (PAG) connectivities on PSP expectancy and drinking severity. The two regions’ connectivity maps and strengths were characterized to assess their shared substrates and net relationship with PSP expectancy. We evaluated mediation and path models to further delineate how mOFC and PAG connectivities interacted through the shared substrates to differentially impact expectancy and alcohol use. During resting, whole-brain regressions showed mOFC connectivity in positive and PAG connectivity in negative association with PSP scores, with convergence in the precentral gyrus (PrCG). Notably, greater PAG-PrCG relative to mOFC-PrCG connectivity strength predicted lower PSP expectancy. During the alcohol cue exposure task, the net strength of the PAG vs. mOFC cue-elicited connectivity with the occipital cortex again negatively predicted PSP expectancy. Finally, mediation and path models revealed that the PAG and mOFC connectivities indirectly and antagonistically modulated problem drinking via their opposing influences on expectancy and craving. Thus, the pain and reward circuits exhibit functional antagonism such that the mOFC connectivity increases expectancy of drinking pleasure whereas the PAG serves to counter that effect.
Similar content being viewed by others
Introduction
Alcohol expectancy is a powerful determinant of drinking behavior1. Anticipation of rewarding alcohol effects, particularly physical and social pleasure (PSP), has been shown to reliably predict immediate consumption and future misuse2,3. Expectancy of drinking pleasure, however, is lessened by anticipated negative consequences (e.g., hangover, impaired judgment, etc.) which can serve to prevent excessive intake and escalation to dependence4,5. Thus, alcohol expectancy is regulated by the balance between two opposing responses to drinking. When biased towards PSP, alcohol expectancy represents a significant risk factor for abuse, as posited by motivational theories of alcohol use6,7.
The involvement of positive and negative alcohol effects in determining expectancy of PSP suggests the recruitment of both reward and pain circuits. Indeed, regions associated with reward processing, especially the medial orbitofrontal cortex (mOFC), have been consistently implicated in both the positive perception and pleasure of drinking. Alcohol administration induced endogenous opioid release in the mOFC8 and the feeling of pleasure9. The mOFC also increases activation to alcohol ingestion and cues10,11 and this activity was predictive of craving ratings in alcoholics12. In contrast, the aversive effects of alcohol elicit pain responses and activate the periaqueductal gray (PAG) of the pain circuit which in turn may play a role in reducing future consumption. Indeed, disruptions of PAG-medial prefrontal cortex connections in mice led to compulsive drinking despite the pairing of foot shock and unpleasant taste with alcohol13. This finding demonstrates the critical role of the PAG in the negative response to drinking. Excessive ethanol intake increased neuronal activity and altered expressions of anxiety-related genes in the PAG14,15, consistent with reports of alcohol withdrawal-induced anxiety and hyperalgesia in humans16 and rodents17,18. Together, such evidence establishes the important function of the mOFC and PAG in assessing the positive and negative effects of alcohol. As reward and pain antagonistically influence alcohol expectancy, their underlying brain circuits may also compete to modulate drinking behavior. Nevertheless, how they interact to regulate expectancy of PSP and alcohol misuse remains unclear.
While brain regions implicated in pain and reward processing play a crucial role in the response to motivationally significant stimuli, they likely work in conjunction with other structures to guide behaviors. For instance, alcohol administration was shown to enhance functional connectivity of multiple domains including the affective19, visual20, motor21, and frontal22 cortices. During noxious thermal stimulation, the dorsolateral prefrontal cortical connectivity with the mOFC increased with heat while its connectivity with the PAG positively predicted opioid release in the PAG during placebo treatment23, potentially reflecting the mechanisms underlying expected pain and pain relief. Thus, it is plausible that the sensory and frontal systems serve as the common substrates for the interaction of reward and pain circuits during the regulation of anticipation, perception, and behavior. Whether such interactions are present in alcohol expectancy or how they may dictate drinking behavior is poorly understood.
Here, we characterized the pain and reward circuits’ antagonistic connectivities in relation to expectancy of PSP and quantify their differential effects on drinking severity. As alcohol expectancy represents a stable psychological construct but one which can be activated or enhanced in response to external cues24, the relationship between expectancy of PSP and brain connectivities was examined during both resting state and during alcohol cue exposure. During resting, we tested the hypothesis that mOFC functional connectivity would be associated with greater expectancy of PSP whereas PAG connectivity would exhibit the opposite pattern. As the two circuits likely showed mutual antagonism, we expected their net connectivity strength to determine the degree of expectancy. We next confirmed the presence of such antagonism during alcohol cue exposure. Finally, models in which the mOFC and PAG interacted to regulate alcohol expectancy, craving, and misuse were evaluated. Together, our findings shed light on how the competing mOFC and PAG impact alcohol misuse via the expectancy of drinking pleasure.
Methods
Participants
One hundred and eighty adult drinkers (85 females; age = 37.7 ± 13.9 years) participated in the study. Subjects provided written informed consent after details of the study were explained in accordance to institute guidelines approved by the Yale Human Investigation Committee.
Participants completed the PSP subscale of the Alcohol Expectancy Questionnaire25 (Supplemental Methods), reporting a PSP score of 19.6 ± 5.9 (mean ± SD) with higher scores indicating greater expectancy. Participants also completed the Alcohol Use Disorder Identification Test (AUDIT)26 with higher scores suggesting greater risk for having or developing an alcohol use disorder. An average AUDIT score of 6.8 ± 7.4 indicated moderate severity of problem drinking27. The 71 participants who performed the alcohol cue craving task further reported their drinking duration (in years) and alcohol craving. In addition, a self-assessment measuring out-of-control drinking behavior on a scale from 0 to 10 (0 = completely in control, 10 = completely out of control) was administered.
Resting and alcohol cue reactivity task
All 180 subjects completed one session of 10-min resting-state fMRI. Following the resting-state run, a subsample of 71 subjects (34 females, age = 36.1 ± 14.0 years) also performed an alcohol cue reactivity (ACR) task in the same fMRI session. The remaining subjects were part of a different study.
In the ACR task (Fig. S1), participants viewed alternating blocks of alcohol-related (e.g., alcoholic drinks, bar scenes, etc.) and neutral (e.g., milk, orange juice, etc.) images. In each block, after a 2-s fixation, six pictures displaying alcohol (alcohol block) or neutral (neutral block) cues were shown for 6 s each. Participants were instructed to view the stimuli and contemplate how they may relate to them. At the end of each block, participants rated their alcohol craving on a scale of 0–10 (0 = no craving at all, 10 = highest craving possible). Participants completed two 9-min runs with each run consisting of six alcohol and six neutral blocks.
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with (TR) = 1900 ms, echo time (TE) = 2.52 ms, bandwidth = 170 Hz/pixel, FOV = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional blood oxygenation level-dependent (BOLD) signals were acquired using multiband imaging (multiband acceleration factor = 3) with a single-shot gradient echo echoplanar imaging sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62◦, field of view = 210 × 210 mm, matrix = 84 × 84, with slice thickness = 2.5 mm and no gap. Data preprocessing steps follow our previous work28 and are reported in Supplementary Methods.
Functional connectivity–resting state
For seed-based resting-state functional connectivity (rsFC), we employed masks of the mOFC (MNI coordinates x = 2, y = 46, z = −8, k = 146) obtained from an imaging meta-analysis examining reward processing29 and the PAG (x = 0, y = −32, z = −9, k = 30) from the Harvard Ascending Arousal Network30 (Fig. S2). The correlation coefficient between the averaged time course of the seed region and that of every other voxel was computed then Fisher’s z transformed for each subject. The Z maps were used in group, random effect analyses in which we conducted whole-brain multiple regressions against the PSP scores, with age and sex as the covariates. All imaging results were examined with the threshold of voxel-level p < 0.001 (uncorrected) in combination with cluster-level p < 0.05 (corrected for family-wise error).
Cue-elicited activations and connectivities—ACR task
A general linear model (GLM) was constructed as in previous work with similar task designs31,32 to differentiate regional activations to alcohol and neutral cues (Supplemental Methods). One-sample t-tests were conducted at the individual subject level to evaluate alcohol vs. neutral cue contrasts.
Cue-elicited connectivity of the PAG and mOFC during alcohol vs. neutral cues was estimated using general psychophysiological interactions (gPPI)33 (Supplemental Methods). We used the same PAG and mOFC seeds as in the rsFC analysis. As the occipital cortex (OC) showed preferential responses to alcohol cues (see “Results”), we examined its cue-elicited connectivity with the PAG and mOFC in a region-of-interest analysis. We extracted parameter estimates which represented their connectivity strength for the contrast alcohol >neutral cues and assessed their relationship with PSP scores, AUDIT scores, and alcohol craving ratings.
Mediation and path analyses
To examine the inter-relationships of PAG, mOFC connectivities, PSP, and AUDIT scores, we conducted mediation analyses34 (Supplemental Methods). For each seed, we considered all six models, with each model featuring the following as the independent variable (X), dependent variable (Y), and mediator (M), respectively: Model 1—functional connectivity, AUDIT scores, and PSP scores; Model 2—AUDIT, connectivity, and PSP; Model 3—AUDIT, PSP, and connectivity; Model 4—PSP, AUDIT, and connectivity; Model 5—connectivity, PSP, and AUDIT; Model 6—PSP, connectivity, and AUDIT.
We also employed path analysis to evaluate how PAG and mOFC connectivity with the precentral gyrus (denoted as PAG/PrCG and mOFC/PrCG from here on) during resting state (see “Results”) as well as with the OC during the ACR task modulated PSP and AUDIT scores. Model fit was assessed with standard fit indices35,36 (Supplemental Methods). For resting-state data, we evaluated the model in which PAG and mOFC connectivities differentially influenced alcohol expectancy which in turn affected problem drinking. An alternative model in which PSP scores influenced AUDIT scores which in turn modulated mOFC/PrCG and PAG/PrCG connectivities was also considered. For alcohol cue-elicited data, we evaluated the model in which difference in connectivity strength of the PAG and mOFC indirectly modulated problem drinking by influencing alcohol expectancy and craving. An alternative model in which PSP indirectly influenced connectivity difference via AUDIT and craving was also considered. Both direct and indirect effects were examined with bootstrapping37 to determine how connectivities from the pain and reward circuits modulated problem drinking and whether these modulations were subjected to mediation effects.
Results
Drinking behavior assessments
All 180 participants reported PSP scores (M ± SD = 19.6 ± 5.9) and AUDIT scores (6.8 ± 7.4), with higher scores indicating greater expectancy and drinking severity. During the ACR task, 71 participants further rated their alcohol craving (3.2 ± 2.7). The self-assessment for out-of-control of the same group yielded an average score of 1.7 ± 2.4. As expected, there were significant positive relationships between the scores of PSP, AUDIT, alcohol craving, and out-of-control drinking assessment (p’s < 0.01, Table S1).
Alcohol expectancy and PAG resting-state connectivity
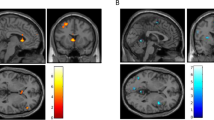
For rsFC, we used the PAG as the seed and performed a whole-brain multiple regression of PAG connectivity using PSP scores as the predictor. Results showed a negative correlation between PSP scores and PAG connectivity with the bilateral precentral gyrus (PrCG), right postcentral gyrus (PoCG), and paracentral lobule (PCL) (Fig. 1a, Table S2). No clusters showed a significant positive correlation between PSP scores and PAG connectivity.
a Whole-brain multiple regression showed a negative correlation between PSP and PAG (far left in green) connectivity with the precentral gyrus (PrCG), postcentral gyrus (PoCG), and paracentral lobule (PCL) (right, blue). b AUDIT scores were positively correlated with PSP scores (left) and negatively correlated with averaged connectivity strength of PAG with PrCG, PoCG, and PCL (right). c Mediation analysis showed two significant models in which PSP bidirectionally mediated the negative relationship between PAG connectivity and AUDIT scores. NB: all partial correlations in this and other figures show regression residuals of parameter estimates of connectivity, PSP, and AUDIT scores after the effects of age and sex had been removed. **p < 0.01.
As PSP scores were negatively correlated with PAG rsFC and both showed a significant relationship with AUDIT scores (Fig. 1b), we used mediation analysis to examine their inter-relationships. PAG rsFC was calculated by averaging the parameter estimates of PAG connectivity with PrCG, PoCG, and PCL as identified by the multiple regression. The results showed two significant models (Fig. 1c, Table S3). In Model 1, PAG connectivity reduced PSP expectancy, which in turn lowered problem drinking: PAG connectivity → PSP → AUDIT. The model showed a significant and full mediation effect after correction for multiple model testing (c − c’ = −2.36, p < 0.001, Supplemental Results). Model 2 (AUDIT → PSP → PAG connectivity) also showed a significant and full mediation effect (c − c’ = −0.017, p < 0.001). None of the other four models was significant (corrected p’s > 0.06). Taken together, enhanced PAG connectivity with the PrCG, PoCG, and PCL was associated with lower drinking severity and this negative relationship was fully and bidirectionally mediated by reduced PSP expectancy.
Alcohol expectancy and mOFC resting-state connectivity
Using the mOFC as the seed, we performed a whole-brain multiple regression of the mOFC rsFC with PSP scores as the predictor. Results showed a positive correlation between PSP scores and mOFC connectivity with the left PrCG (Fig. 2a, Table S2). No significant negative correlation for mOFC connectivity and PSP scores was found.
a Whole-brain multiple regression showed positive correlation between PSP scores and the mOFC (far left in green) connectivity with the left precentral gyrus (PrCG) (right, red). b mOFC/PrCG connectivity strength showed a significant positive correlation with PSP (left) and a near significant positive correlation with AUDIT scores (right). c Mediation analysis showed two significant models in which PSP bidirectionally mediated the positive relationship between mOFC/PrCG connectivity and AUDIT scores. Note: mOFC/PrCG denotes mOFC connectivity with PrCG. **p < 0.01.
As with the PAG connectivity, we examined the inter-relationship between mOFC/PrCG connectivity, PSP, and AUDIT scores in a mediation analysis. Medial OFC/PrCG connectivity showed a significant positive correlation with PSP as expected (Fig. 2b left) and a near significant correlation with AUDIT scores (p = 0.08, Fig. 2b right). There was a significant mediation effect in Model 1 (mOFC connectivity → PSP → AUDIT) after correction for multiple model testing (c − c’ = 1.64, p < 0.001) (Fig. 2c, Table S4). However, while path coefficient c was significantly weakened after accounting for the mediating effect of PSP, the relationship between mOFC connectivity and AUDIT scores only reached near significance (p = 0.10). It is worth noting that the significance of this relationship is not considered a requirement for mediation analysis38. Model 2 (AUDIT → PSP → mOFC connectivity) also showed a significant mediation effect (c − c’ = 0.019, p = 0.001). Again, the relationship between mOFC connectivity and AUDIT scores was only trending toward significance (p = 0.07). None of the remaining models was significant (p’s > 0.50). Thus, elevated mOFC connectivity with the PrCG was associated with increased drinking severity and this relationship was bidirectionally mediated by heightened PSP expectancy.
Opposing effects of PAG and mOFC rsFC on alcohol expectancy and misuse
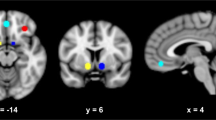
To determine whether the rsFC patterns of the PAG and mOFC shared common substrates, we examined the PAG and mOFC connectivity maps which overlapped in the left PrCG (Fig. 3a). Thus, mOFC/PrCG connectivity exhibited a positive relationship with PSP scores whereas PAG/PrCG connectivity showed a negative relationship (Fig. S3).
a PSP scores were negatively correlated with PAG rsFC (blue) but positively correlated with mOFC rsFC (red) and the two connectivity maps overlapped in the left PrCG (yellow). b PSP scores were negatively predicted by the difference in connectivity strength between PAG/PrCG and mOFC/PrCG, indicating the stronger the PAG/PrCG relative to mOFC/PrCG connectivity, the lower PSP expectancy. c Path analysis showed PAG and mOFC rsFC with the PrCG indirectly and antagonistically influenced drinking severity through their modulation of expectancy. PAG/PrCG rsFC lessened drinking severity by reducing PSP expectancy (blue arrow). In contrast, mOFC/PrCG rsFC exacerbated drinking severity by enhancing PSP expectancy (red arrow). β’s represent path coefficients. **p < 0.01.
To quantify the opposing effects of PAG and mOFC rsFC on alcohol expectancy, we first calculated the difference in strength of their connectivity with the overlapping PrCG (i.e., [PAG connectivity with PrCG] minus [mOFC connectivity with PrCG]). This net connectivity strength significantly and negatively predicted PSP scores (r = −0.45, p < 0.001, Fig. 3b). Thus, the stronger the PAG/PrCG relative to mOFC/PrCG connectivity, the lower the PSP expectancy.
We next employed path analysis to further characterize the antagonism of PAG and mOFC rsFC with the PrCG in modulating expectancy and drinking severity in the same model. We tested the scenario in which PAG and mOFC connectivities indirectly influenced AUDIT scores via their opposing effects on PSP expectancy (Fig. 3c). Parameter estimates of the PAG and mOFC rsFC with the PrCG were used as the two exogenous variables. The model showed a good fit (Fit indices: RMSEA = 0.00 [90% CI: 0.00 0.11], χ2/df = 0.33, SRMR = 0.02, and CFI = 1.00). PSP scores were negatively modulated by PAG/PrCG connectivity (β = −0.21, p = 0.004) but positively modulated by mOFC/PrCG connectivity (β = 0.24, p = 0.001). Bootstrapping assessing indirect effects showed significant mediation effects of PSP on the relationship of AUDIT scores with PAG as well as with mOFC connectivities. Specifically, PAG/PrCG connectivity decreased problem drinking through reducing PSP expectancy (β = −0.12, p = 0.001, full mediation). Conversely, mOFC/PrCG connectivity increased problem drinking through enhancing PSP expectancy (β = 0.11, p = 0.004, full mediation). Thus, problem drinking was positively modulated by the connectivity of the reward circuit but negatively modulated by that of the pain circuit and these modulations were achieved indirectly via PSP expectancy.
We also considered the model in which PSP scores differentially modulated PAG and mOFC connectivity via AUDIT scores (Fig. S4). The model showed a good fit (Fit indices: RMSEA = 0.00, χ2/df = 0.245, SRMR = 0.333, and CFI = 1.00), indicating the direction of influence between PAG as well as mOFC connectivity and expectancy may be bidirectional.
The alternative model in which PSP scores influenced AUDIT scores which in turn modulated PAG and mOFC connectivities showed a poor fit (Fit indices: RMSEA = 0.23, χ2/df = 10.80, SRMR = 0.10, and CFI = 0.73, Fig. S4) and was not considered further.
Cue-related PAG and mOFC connectivities, alcohol expectancy, and problem drinking
For the ACR task, we identified the brain regions with preferential responses to alcohol cues. The contrast alcohol > neutral cues showed significant activations in the mOFC, bilateral OC, posterior cingulate cortex, and left superior frontal gyrus (Fig. 4a, Table S5). The reverse contrast neutral >alcohol cues yielded significant activation in the parieto-occipital sulcus.
a During the ACR task, alcohol relative to neutral cues elicited activations in the mOFC, superior frontal gyrus (SFG), and a cluster containing the bilateral occipital cortices (OC) and posterior cingulate cortex (PCC) (red). The reverse contrast showed significant activation in the parieto-occipital sulcus (blue). b PSP scores were negatively correlated with the difference in connectivity strength between PAG/OC and mOFC/OC during alcohol cue processing, indicating the stronger the PAG/OC relative to mOFC/OC connectivity, the lower the PSP expectancy. c Path analysis showed a significant model in which PAG vs. mOFC connectivity strength difference indirectly reduced problem drinking by lowering PSP, which in turn lowered alcohol craving. Solid and dotted lines show significant and non-significant paths, respectively. *p < 0.05, **p < 0.01.
As the OC is shown here and elsewhere10 to exhibit robust responses to alcohol cues, we next determined whether the cue-elicited connectivity of the OC with the PAG and mOFC modulated PSP expectancy during alcohol cue exposure. Here, the OC was defined from the alcohol > neutral cue contrast. PPI model was estimated with the PAG and mOFC as the seeds for the connectivity with the OC during alcohol vs. neutral cues. Parameter estimates of the PAG connectivity with OC and mOFC connectivity with OC were then extracted for the gPPI contrast alcohol > neutral cues. We then examined their relative strength in relation to alcohol expectancy. The difference in connectivity strength of the PAG and mOFC with the OC (i.e., [PAG connectivity with OC] minus [mOFC connectivity with OC]) was significantly and negatively correlated with PSP scores (r = −0.31, p < 0.01, Fig. 4b). Thus, stronger PAG connectivity strength relative to the mOFC connectivity, both with the OC, predicted lower PSP expectancy during alcohol cue exposure. As with the rsFC, the finding indicates competing roles of PAG and mOFC connectivities in modulating alcohol expectancy.
Alcohol craving ratings showed a significant relationship with both PSP (r = 0.34, p = 0.004) and AUDIT (r = 0.38, p = 0.001) scores. Thus, we next examined how the difference in connectivity strength between PAG/OC and mOFC/OC may influence alcohol expectancy, craving, and misuse in a path analysis (Fig. 4c). We tested the model in which PAG vs. mOFC connectivity strength difference indirectly influenced AUDIT scores by negatively modulating PSP and alcohol craving. The model showed a good fit (Fit indices: RMSEA = 0.00 [90% CI: 0.00 0.24], χ2/df = 0.17, SRMR = 0.013, and CFI = 1.00). Specifically, PAG vs. mOFC connectivity strength difference significantly reduced PSP scores (β = −0.31, p = 0.006) which in turn affected alcohol craving (β = 0.36, p = 0.002). Both PSP and craving rating then increased AUDIT scores (p’s ≤ 0.011). Thus, PAG relative to mOFC connectivity lowered PSP expectancy, leading to attenuated alcohol craving, and subsequently decreased problem drinking.
We also considered the model in which PSP modulated PAG vs. mOFC connectivity difference via AUDIT scores (Fig. S5). Results showed that the model showed a good fit (Fit indices: RMSEA = 0.00, χ2/df = 0.245, SRMR = 0.015, and CFI = 1.00), indicating the direction of influence between PAG vs. mOFC connectivity and expectancy of drinking pleasure may be bidirectional.
The alternative model in which PSP expectancy indirectly influenced PAG/mOFC connectivity difference via problem drinking and craving showed a poor fit (Fit indices: RMSEA = 0.29, χ2/df = 6.92, SRMR = 0.08, and CFI = 0.81, Fig. S5) and was not considered further. Finally, defining the OC using a more stringent threshold (Fig. S6) did not materially change the results.
Discussion
We identified the competing modulation of the reward and pain circuits on alcohol expectancy by characterizing functional connectivities of the mOFC and PAG during resting state and during alcohol cue exposure. Our findings were threefold. First, elevated rsFC of the mOFC was associated with heightened expectancy of drinking pleasure which in turn predicted greater drinking severity. In contrast, PAG connectivity exhibited the opposite influence. Second, the mOFC and PAG rsFC maps converged in the precentral gyrus (PrCG). Through the PrCG, the mOFC and PAG indirectly and antagonistically modulated drinking behavior by exerting opposing effects on alcohol expectancy. Further, their net connectivity strength predicted the degree of expectancy. Finally, during alcohol cue exposure, the PAG and mOFC cue-elicited connectivities, both involving the same visual area, again exhibited competing modulation on alcohol expectancy and drinking severity. Together, these findings support the functional antagonism between the pain and reward circuits such that PAG connectivity protects against problem drinking while mOFC connectivity serves as a risk factor for alcohol misuse.
The PAG plays a central role in nociception, responding to both anticipated39 and perceived40 pain. In problem drinking, the PAG has been implicated in hyperalgesia41,42,43. Alcohol dependence frequently produces sustained negative emotional states and chronic pain possibly through the alcohol-induced alterations of the brain mechanisms supporting stress response and pain transmission, both of which involve the PAG44. Structural abnormalities of the PAG in dependent individuals were previously observed45, corroborating the reports of dysphoria and increased pain sensitivity in this population46. In rodents, alcohol administration and binge drinking not only elevated neuronal activity47 but also induced changes in N-methyl-D-aspartate and serotonin receptor gene expressions in the PAG, likely increasing susceptibility to pain, fear, and anxiety15. In addition, alcohol withdrawal in rats raised concentrations of pain signal-related nitric oxide in the PAG14. Collectively, previous evidence suggests the PAG responds to the aversive effects of alcohol use, thus countering the expectancy of drinking pleasure.
The PAG interacts with other brain structures to regulate motivated behaviors48 including the motor/premotor cortex which is anatomically connected with the PAG49 and responsive to both actual and anticipated pain50,51. In particular, the connectivity between the PAG and the PrCG, PoCG, and PCL was shown to increase with thermal pain intensity52, mirroring the current rsFC finding of the PAG. It is plausible that the negative modulation of PAG connectivity on pleasure expectancy and problem drinking was reflective of the PAG’s response to anticipated aversive consequences, leading to the suppression of alcohol-seeking behavior. To our knowledge, our imaging evidence is the first to substantiate the role of the pain circuit in deterring problem drinking in humans.
While alcohol misuse can produce painful effects, many individuals continue to drink to excess, reflecting a bias towards the anticipated pleasure derived from drinking. The mOFC has been implicated in supporting such rewarding effects of alcohol and other substances53. Alcohol odor and visual cues elicit activation of the mOFC both in healthy and dependent individuals54,55 and this mOFC activity positively predicts subsequent relapse in the latter group56. Reduced mOFC rsFC with the amygdala, a subcortical hub of the saliency circuit, has been associated with lower alcohol consumption in adolescence57. The mOFC, therefore, is involved not only in the pleasant sensation of alcohol consumption but also in the positive perception of drinking, consistent with the current work associating mOFC connectivity with heightened expectancy of pleasure.
The opposite relationships of PAG and mOFC connectivities with alcohol expectancy indicate antagonistic influences of the reward and pain circuits. Past behavioral investigations have underlined the competing roles of expectations for aversive and rewarding outcomes in alcohol consumption. Anticipated negative consequences discourage whereas anticipated pleasure facilitates alcohol use1,2,58. As such, their relative strengths determine drinkers’ net expectancy of drinking pleasure and subsequent alcohol-seeking behavior59. One likely engages in alcohol use if the perceived benefits from drinking outweighs the perceived costs. While the competing nature of pain and pleasure in alcohol use has long been acknowledged60, the neural basis underlying such competition was poorly understood. Our finding that the difference in connectivity strength of the two systems predicted the degree of pleasure expectancy represents new and robust evidence delineating how the reward and pain circuits interact to regulate drinking.
During resting state, the functional antagonism of the reward and pain circuits involved the PrCG which may serve as their shared target to influence drinking behavior. In alcohol dependent individuals, the PrCG exhibited elevated activation to alcohol cues and craving ratings61,62 and this activation was reduced following cue-exposure extinction treatment63. A more direct relationship between PrCG and alcohol expectancy was demonstrated by the association of increased gray matter volume and event-related potentials of the PrCG with heightened positive alcohol expectancy64,65. In contrast, PrCG activity has also been associated with risk avoidance and reduced problem drinking66, indicating the region’s complex role in alcohol use. Dictating motor outputs, the PrCG may enable behavioral responses to motivationally significant events67. Thus, signals from the mOFC and PAG likely help drive PrCG in facilitating approach or avoidance in alcohol seeking, respectively.
During alcohol cue exposure, we again observed the opposing effects of the PAG and mOFC but with the involvement of the OC. While the recruitment of the visual cortex may not be surprising given its robust activation to alcohol cues10 and anatomical connections with both mOFC68 and PAG69, the current findings suggest a more direct role of the OC in shaping drinking behavior. Previous theoretical work proposes that the visual cortex links perceptual processing of drug cues to the psychophysiological responses to actual consumption70. Accordingly, the interaction of the OC with the motivational circuits helps enhance the incentive salience of alcohol cues. In the current study, OC/PAG and OC/mOFC cue-elicited connectivity potentially signaled the negative and positive values of alcohol use, respectively. Their net strength, as a result, likely determines the prevailing affective response that underlines alcohol expectancy. Our path analysis additionally showed the modulations of the two circuits on alcohol craving and expectancy which in turns impacted problem drinking. Such evidence suggests a potential pathway connecting affective processing, perception, craving, and alcohol consumption. Taken together, the current work supports the antagonistic effects of the reward and pain circuits and delineates the involvement of the motor and visual cortices in titrating the expected gains and costs of drinking to regulate alcohol consumption.
Motivational models of alcohol use propose the role of expectancy as a mediator of various risk factors and problem drinking6,7. Corroborating evidence shows that trait anxiety71, sensation-seeking72, and family history of alcoholism73 all impact expectancy which in turn predicts alcohol use. As such, alcohol expectancy represents a common pathway through which distal determinants exert influences on drinking behavior74. Our results from path and mediation analyses support this conceptual framework. Furthermore, the evidence that the PAG plays a mitigating role whereas the mOFC plays a facilitating one in relation to alcohol misuse extends current understanding of risk factors. Individual differences in connectivity strength of the reward relative to pain systems are shown here to potentially be an additional measure of biological vulnerability for problem drinking.
It is worth noting that our models also suggested that expectancy of drinking pleasure can influence PAG and mOFC connectivities, thus making the relationship between expectancy and reward/pain circuits bidirectional. Specifically, expected pleasure can enhance the reward response to alcohol use and dampen the pain circuit. While such relationship requires further investigation, this interpretation is in line with the notion that substance abuse, including alcohol, can lead to the phenomenon in which drugs “hijack” the brain’s reward system75. Problem drinkers seeking stimulation may become increasingly driven by alcohol rewarding effects, even in the face of negative consequences, thus ensuring that the imbalance between pleasure and pain response traps drinkers in a vicious circle.
Conclusions
Drinking, as with other motivated behaviors, is regulated by the expected gains and costs. Motivational models of alcohol use posit opposite impacts of alcohol expectancies on drinking. Expecting rewarding effects of alcohol promotes alcohol use whereas expecting adverse consequences deters individuals from drinking. Here, we demonstrate how the pain and reward circuit connectivities modulate problem drinking via mutually antagonistic influences on alcohol expectancy. These findings suggest a neural basis of the alcohol expectancy which can serve as markers to predict drinking trajectory and inform treatment designs.
References
Brown, S. A., Goldman, M. S. & Christiansen, B. A. Do alcohol expectancies mediate drinking patterns of adults? J. Consult Clin. Psychol. 53, 512–519 (1985).
Hasking, P., Lyvers, M. & Carlopio, C. The relationship between coping strategies, alcohol expectancies, drinking motives and drinking behaviour. Addict. Behav. 36, 479–487 (2011).
Wardell, J. D., Read, J. P., Curtin, J. J. & Merrill, J. E. Mood and implicit alcohol expectancy processes: predicting alcohol consumption in the laboratory. Alcohol Clin. Exp. Res. 36, 119–129 (2012).
King, A. C., De Wit, H., McNamara, P. J. & Cao, D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch. Gen. Psychiatry 68, 389–399 (2011).
Jones, B. T., Corbin, W. & Fromme, K. Conceptualizing addiction: a review of expectancy theory and alcohol consumption. Addiction 96, 57–72 (2001).
Cox, W. M. & Klinger, E. A motivational model of alcohol use. J. Abnorm. Psychol. 97, 168–180 (1988).
Cooper, M. L., Frone, M. R., Russell, M. & Mudar, P. Drinking to Regulate positive and negative emotions: a motivational model of alcohol use. J. Pers. Soc. Psychol. 69, 990–1005 (1995).
Mitchell, J. M. et al. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl. Med. 4, 1–9 (2012).
Volpicelli, J. R., Watson, N. T., King, A. C., Sherman, C. E. & O’brien, C. P. Effect of naltrexone on alcohol ‘high’ in alcoholics. Am. J. Psychiatry 152, 613–615 (1995).
Schacht, J. P., Anton, R. F. & Myrick, H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 18, 121–133 (2013).
Kareken, D. A. et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage 50, 267–276 (2010).
Myrick, H. et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29, 393–402 (2004).
Siciliano, C. A. et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012 (2019).
Long, C., Yang, L., Faingold, C. L. & Steven Evans, M. Excitatory amino acid receptor-mediated responses in periaqueductal gray neurons are increased during ethanol withdrawal. Neuropharmacology 52, 802–811 (2007).
Mcclintick, J. N. et al. Gene expression changes in glutamate and GABA-A receptors, neuropeptides, ion channels, and cholesterol synthesis in the periaqueductal gray following binge-like alcohol drinking by adolescent alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 40, 955–968 (2016).
Jochum, T., Boettger, M. K., Burkhardt, C., Juckel, G. & Bär, K. J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain 14, 713–718 (2010).
Avegno, E. M. et al. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J. Neurosci. 38, 7761–7773 (2018).
Bonassoli, V. T., Contardi, E. B., Milani, H. & De Oliveira, R. M. W. Effects of nitric oxide synthase inhibition in the dorsolateral periaqueductal gray matter on ethanol withdrawal-induced anxiety-like behavior in rats. Psychopharmacology 228, 487–498 (2013).
Bjork, J. M. & Gilman, J. M. The effects of acute alcohol administration on the human brain: insights from neuroimaging. Neuropharmacology 84, 101–110 (2014).
Esposito, F. et al. Alcohol increases spontaneous BOLD signal fluctuations in the visual network. Neuroimage 53, 534–543 (2010).
Shokri-Kojori, E., Tomasi, D., Wiers, C. E., Wang, G.-J. J. & Volkow, N. D. Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Mol. Psychiatry 22, 1–11 (2016).
Khalili-Mahani, N. et al. Effects of morphine and alcohol on functional brain connectivity during ‘resting state’: a placebo-controlled crossover study in healthy young men. Hum. Brain Mapp. 33, 1003–1018 (2012).
Wager, T. D., Scott, D. J. & Zubieta, J. K. Placebo effects on human μ-opioid activity during pain. Proc. Natl Acad. Sci. USA 104, 11056–11061 (2007).
Oei, T. P. & Baldwin, A. R. Expectancy theory: a two-process model of alcohol use and abuse. J. Stud. Alcohol 55, 525–534 (1994).
George, W. H. et al. A revised Alcohol Expectancy Questionnaire: factor structure confirmation, and invariance in a general population sample. J. Stud. Alcohol 56, 177–185 (1995).
Saunders, J., Assland, O., Babor, T., De la fuente, J. & Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 701–904 (1993).
Donovan, D. M., Kivlahan, D. R., Doyle, S. R., Longabaugh, R. & Greenfield, S. F. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction 101, 1696–1704 (2006).
Le, T. M. et al. The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int J. Obes. https://doi.org/10.1038/s41366-019-0496-8 (2019).
Bartra, O., McGuire, J. T. & Kable, J. W. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427 (2013).
Edlow, B. L. et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 71, 531–546 (2012).
Le, T. M., Zhornitsky., S., Wang., W., Zhang, S. & Li, C. R. Problem drinking alters gray matter volume and food cue responses of the lateral orbitofrontal cortex. Addict. Biol. e12857, 1–10 (2019).
Zhornitsky, S. et al. Alcohol expectancy and cerebral responses to cue-elicited craving in adult nondependent drinkers. Biol Psychiatry Cogn Neurosci. Neuroimaging 4, 1–12 (2018).
McLaren, D. G., Ries, M. L., Xu, G. & Johnson, S. C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61, 1277–1286 (2012).
MacKinnon, D. P., Fairchild, A. J. & Fritz, M. S. Mediation analysis. Annu Rev. Psychol. 58, 593–614 (2007).
Hu, L.-T. & Bentler, P. M. In Structural equation modeling: Concepts, issues, and applications. (ed. Hoyle, R. H.), 76–99 (Sage Publications, Inc: Thousand Oaks, CA, US, 1995).
Chen, F., Curran, P. J., Bollen, K. A., Kirby, J. & Paxton, P. An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Socio. Methods Res. 36, 462–494 (2008).
Mahmoud, A. B. & Grigoriou, N. When empathy hurts: modelling university students’ word of mouth behaviour in public vs. private universities in Syria. High. Educ. Q. 71, 369–383 (2017).
Shrout, P. E. & Bolger, N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7, 422–445 (2002).
Wager, T. D. et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 303, 1162–1167 (2004).
Sprenger, C., Finsterbusch, J. & Büchel, C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical fMRI study. J. Neurosci. 35, 4248–4257 (2015).
Robins, M. T., Heinricher, M. M. & Ryabinin, A. E. From pleasure to pain, and back again: the intricate relationship between alcohol and nociception. Alcohol Alcohol 54, 625–638 (2019).
Lovick, T. A. Pro-nociceptive action of cholecystokinin in the periaqueductal grey: a role in neuropathic and anxiety-induced hyperalgesic states. Neurosci. Biobehav. Rev. 32, 852–862 (2008).
Heinricher, M. M., Martenson, M. E. & Neubert, M. J. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain 110, 419–426 (2004).
Egli, M., Koob, G. F. & Edwards, S. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev. 36, 2179–2192 (2012).
Zahr NM. Structural and Microstructral Imaging of the Brain in Alcohol Use Disorders. 1st edn. (Elsevier B.V., 2014).
Gatch, M. B. Ethanol withdrawal and hyperalgesia. Curr. Drug Abus. Rev. 2, 41–50 (2009).
Li, C., McCall, N. M., Lopez, A. J. & Kash, T. L. Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons. Alcohol 47, 279–287 (2013).
Benarroch, E. E. Periaqueductal gray: an interface for behavioral control. Neurology 78, 210–217 (2012).
An, X., Bandler, R., Öngür, D. & Price, J. L. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 401, 455–479 (1998).
Palermo, S., Benedetti, F., Costa, T. & Amanzio, M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum. Brain Mapp. 36, 1648–1661 (2015).
Kanda, M. et al. Primary somatosensory cortex is actively involved in pain processing in human. Brain Res. 853, 282–289 (2000).
Linnman, C., Beucke, J. C., Jensen, K. B., Gollub, R. L. & Kong, J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain 153, 444–454 (2012).
Volkow, N. D. & Fowler, J. S. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex 10, 318–25 (2000).
Bragulat, V. et al. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin. Exp. Res. 32, 1124–1134 (2008).
Kim, S. M., Han, D. H., Min, K. J., Kim, B. N. & Cheong, J. H. Brain activation in response to craving- and aversion-inducing cues related to alcohol in patients with alcohol dependence. Drug Alcohol Depend. 141, 124–131 (2014).
Blaine, S. K., Seo, D. & Sinha, R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict. Biol. 22, 468–478 (2017).
Peters, S., Jolles, D. J., Duijvenvoorde, A. C. K. V., Crone, E. A. & Peper, J. S. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology 53, 117–126 (2015).
Leigh, B. C. & Stacy, A. W. Alcohol expectancies and drinking in different age groups. Addiction 99, 215–227 (2004).
Collins, R. L., Lapp, W. M., Emmons, K. M. & Isaac, L. M. Endorsement and strength of alcohol expectancies. J. Stud. Alcohol 51, 336–342 (1990).
Goldman, M. S. The alcohol expectancy concept: applications to assessment, prevention, and treatment of alcohol abuse. Appl. Prev. Psychol. 3, 131–144 (1994).
Feldstein Ewing, S. W., Filbey, F. M., Sabbineni, A., Chandler, L. D. & Hutchison, K. E. How psychosocial alcohol interventions work: a preliminary look at what fMRI can tell us. Alcohol Clin. Exp. Res. 35, 643–651 (2011).
Park, M. S. et al. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol 42, 417–422 (2007).
Vollstädt-Klein, S. et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol. Psychiatry 69, 1060–1066 (2011).
Ide, J. S. et al. Gray matter volume correlates of global positive alcohol expectancy in non-dependent adult drinkers. Addict. Biol. 19, 895–906 (2014).
Deckel, A. W., Hesselbrock, V. & Bauer, L. Relationship between alcohol‐related expectancies and anterior brain functioning in young men at risk for developing alcoholism. Alcohol Clin. Exp. Res. 19, 476–481 (1995).
Claus, E. D. & Hutchison, K. E. Neural mechanisms of risk taking and relationships with hazardous drinking. Alcohol Clin. Exp. Res. 36, 408–416 (2012).
Thoenissen, D., Zilles, K. & Toni, I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J. Neurosci. 22, 9024–9034 (2002).
Burks, J. D. et al. Anatomy and white matter connections of the orbitofrontal gyrus. J. Neurosurg. 128, 1865–1872 (2018).
Redgrave, P. & Dean, P. in The Midbrain Periaqueductal Gray Matter: Functional, Anatomical, and Neurochemical Organization. (eds Depaulis, A, & Bandler, R.) 199–209 (Springer US, Boston, MA, 1991).
Robinson, T. E. & Berridge, K. C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 18, 247–291 (1993).
Cooper, M. L., Russell, M., Skinner, J. B., Frone, M. R. & Mudar, P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J. Abnorm. Psychol. 101, 139–152 (1992).
Urbán, R., Kökönyei, G. & Demetrovics, Z. Alcohol outcome expectancies and drinking motives mediate the association between sensation seeking and alcohol use among adolescents. Addict. Behav. 33, 1344–1352 (2008).
Mann, L. M. L., Chassin, L. & Sher, K. J. Alcohol expectancies and the risk for alcoholism. J. Consult. Clin. Psychol. 55, 411–417 (1987).
Cooper, M. L. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol. Assess. 6, 117–128 (1994).
Nesse, R. M. B. & Berridge, K. C. Psychoactive drug use in evolutionary perspective. Science 278, 63–66 (1997).
Acknowledgements
This study was supported by NIH grant AA021449. The NIH is otherwise not involved in the conceptualization of the study, data collection and analysis, or in the decision to publish the current results.
Author information
Authors and Affiliations
Contributions
C-.S.R.L. and T.M.L. designed the study, S.Z. and S.Z. collected the data, T.M.L. analyzed the data, all authors wrote the paper. All authors approve this manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le, T.M., Zhornitsky, S., Zhang, S. et al. Pain and reward circuits antagonistically modulate alcohol expectancy to regulate drinking. Transl Psychiatry 10, 220 (2020). https://doi.org/10.1038/s41398-020-00909-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-00909-z
This article is cited by
-
Cortical thickness of the inferior parietal lobule as a potential predictor of relapse in men with alcohol dependence
Brain Imaging and Behavior (2023)
-
Neural correlates of individual variation in two-back working memory and the relationship with fluid intelligence
Scientific Reports (2021)
-
The Periaqueductal Gray and Its Extended Participation in Drug Addiction Phenomena
Neuroscience Bulletin (2021)